- Главная
- Разное
- Образование
- Спорт
- Естествознание
- Природоведение
- Религиоведение
- Французский язык
- Черчение
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, фоны, картинки для презентаций
- Экология
- Экономика
Презентация, доклад по химии на тему: Сера на английском языке
Содержание
- 1. Презентация по химии на тему: Сера на английском языке
- 2. General propertiesAlternative name sulphur
- 3. Sulfur or sulphur is a chemical element with symbol S and atomic number 16. It
- 4. Sulfur is the tenth most common element
- 5. Physical properties When burned, sulfur melts to
- 6. Phase at STP solidMelting point
- 7. Chemical properties Sulfur burns with a blue
- 8. Allotropes Amorphous or "plastic" sulfur is produced by
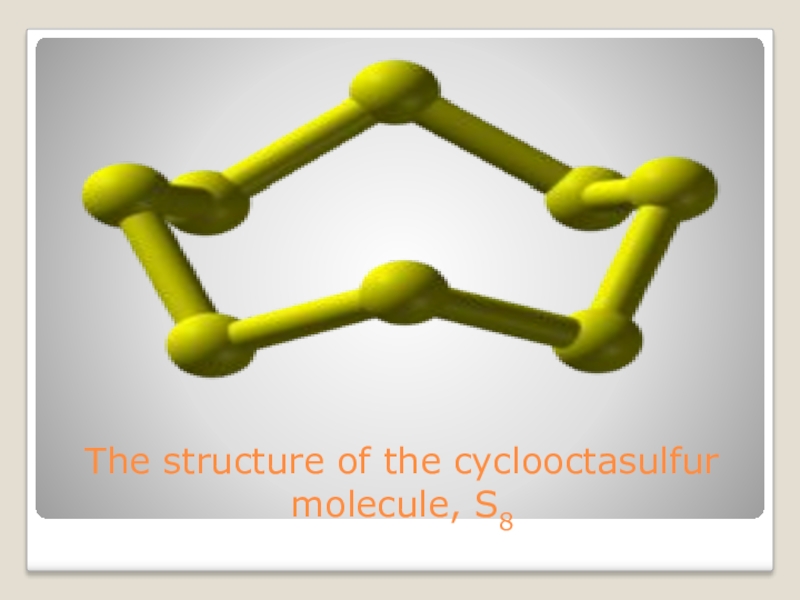
- 9. The structure of the cyclooctasulfur molecule, S8
General propertiesAlternative name sulphur (British spelling)Appearancelemon yellow sintered microcrystals Standard atomicStandard atomic weight (Ar, standard) [32.059, 32.076]
Слайд 2General properties
Alternative name sulphur (British spelling)
Appearancelemon yellow
sintered microcrystals
Standard atomicStandard atomic
weight (Ar, standard) [32.059, 32.076]
conventional: 32.06
Standard atomicStandard atomic
weight (Ar, standard) [32.059, 32.076]
conventional: 32.06
Слайд 3Sulfur or sulphur is a chemical element with symbol S and atomic number 16.
It is abudant, multivalent, and nonmetalic.
Under normal
conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8.
Elemental sulfur is a bright yellow crystalline solid at room temperature.
Chemically, sulfur reacts with all elements except for gold, platinum, iridium, tellurium, and the noble gases.
Elemental sulfur is a bright yellow crystalline solid at room temperature.
Chemically, sulfur reacts with all elements except for gold, platinum, iridium, tellurium, and the noble gases.
Слайд 4Sulfur is the tenth most common element by mass in the
universe, and the fifth most common on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and Egypt. Today, almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.
Слайд 5Physical properties
When burned, sulfur melts to a blood-red liquid and emits
a blue flame that is best observed in the dark.
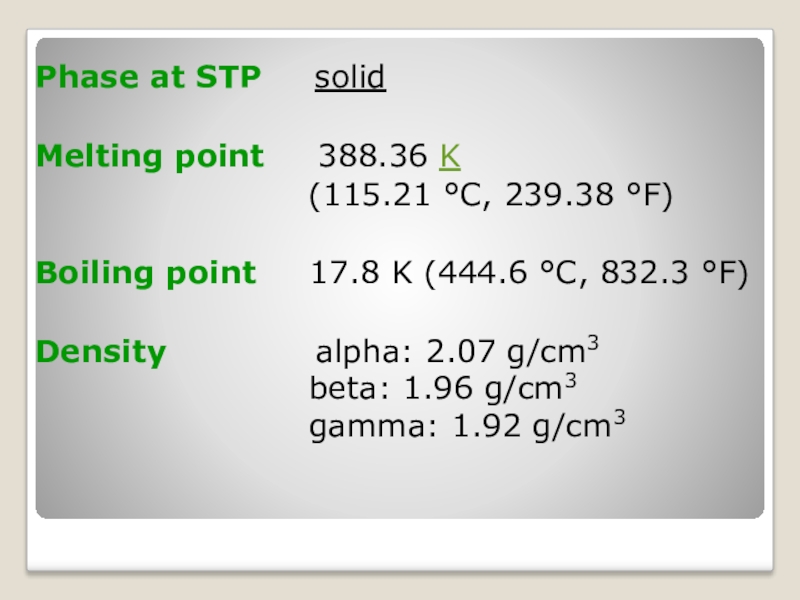
Слайд 6Phase at STP solid
Melting point 388.36 K
(115.21 °C, 239.38 °F)
Boiling point 17.8 K (444.6 °C, 832.3 °F)
Density alpha: 2.07 g/cm3 beta: 1.96 g/cm3 gamma: 1.92 g/cm3
Boiling point 17.8 K (444.6 °C, 832.3 °F)
Density alpha: 2.07 g/cm3 beta: 1.96 g/cm3 gamma: 1.92 g/cm3
Слайд 7Chemical properties
Sulfur burns with a blue flame with formation of sulfur dioxide,
which has a suffocating and irritating odor. Sulfur is insoluble in water but soluble in carbon disulfide and, to a lesser extent, in other nonpolar organic solvents, such as benzene and toluene.
Слайд 8Allotropes
Amorphous or "plastic" sulfur is produced by rapid cooling of molten sulfur—for
example, by pouring it into cold water. X-ray crystallography studies show that the amorphous form may have a helical structure with eight atoms per turn. The long coiled polymeric molecules make the brownish substance elastic, and in bulk this form has the feel of crude rubber. This form is metastable at room temperature and gradually reverts to crystalline molecular allotrope, which is no longer elastic. This process happens within a matter of hours to days, but can be rapidly catalyzed.