- Главная
- Разное
- Образование
- Спорт
- Естествознание
- Природоведение
- Религиоведение
- Французский язык
- Черчение
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, фоны, картинки для презентаций
- Экология
- Экономика
Презентация, доклад по химии на тему Кристаллический клетки (8,9,10,11 класс)
Содержание
- 1. Презентация по химии на тему Кристаллический клетки (8,9,10,11 класс)
- 2. INDEXINTRODUCTIONMETALLIC BONDSIONIC SOLIDSNETWORK SOLIDSDIPOLE–DIPOLE FORCESVAN DER WAALS FORCESHYDROGEN BONDS
- 3. INTRODUCTIONIn the solid or liquid phase molecules
- 4. METALLIC BONDSMetals are solid at room temperature
- 5. Attraction force between the negatively charged “sea
- 6. Слайд 6
- 7. Metals are good conductors of heat and
- 8. Слайд 8
- 9. Metallic bond is better maan!
- 10. Слайд 10
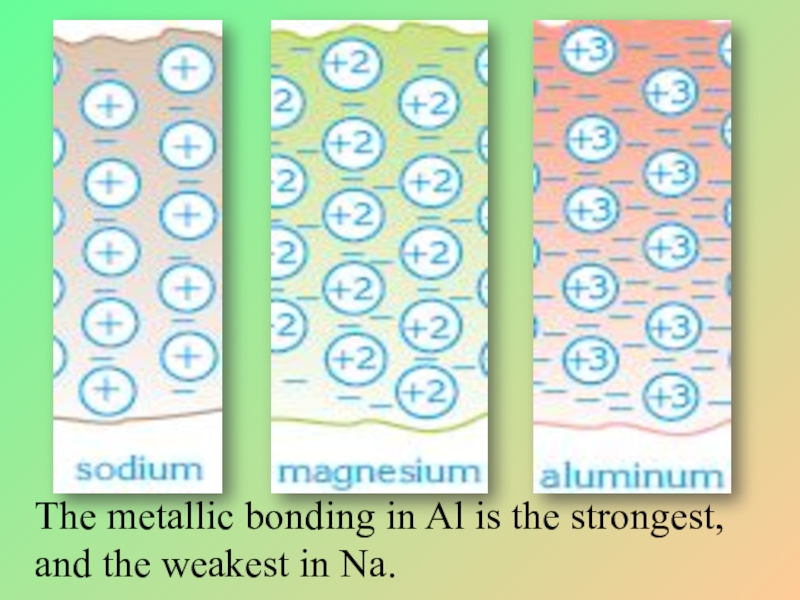
- 11. The metallic bonding in Al is the strongest, and the weakest in Na.
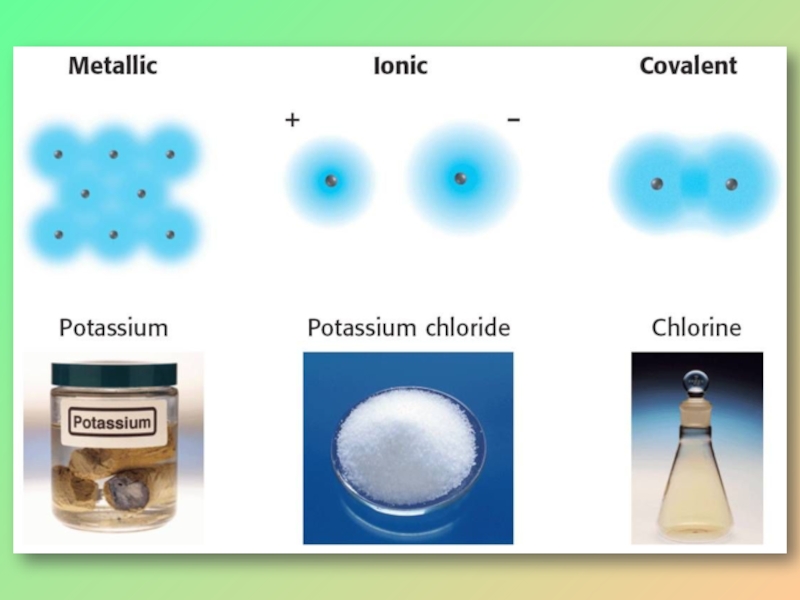
- 12. IONIC SOLIDSWhen metal and nonmetal atoms come
- 13. .Ionic solids do not have a molecular
- 14. NETWORK SOLIDSIn some covalent substances, known as
- 15. DiamondEach carbon atom is covalently bonded to
- 16. Silicon carbide SiC is another network solid.
- 17. Diamond and silicon carbide are nonconductors of electricity and have very high melting points.
- 18. GraphiteEach carbon atom is bonded to three
- 19. Слайд 19
- 20. DIPOLE–DIPOLE FORCESThe atrractions of the positive and
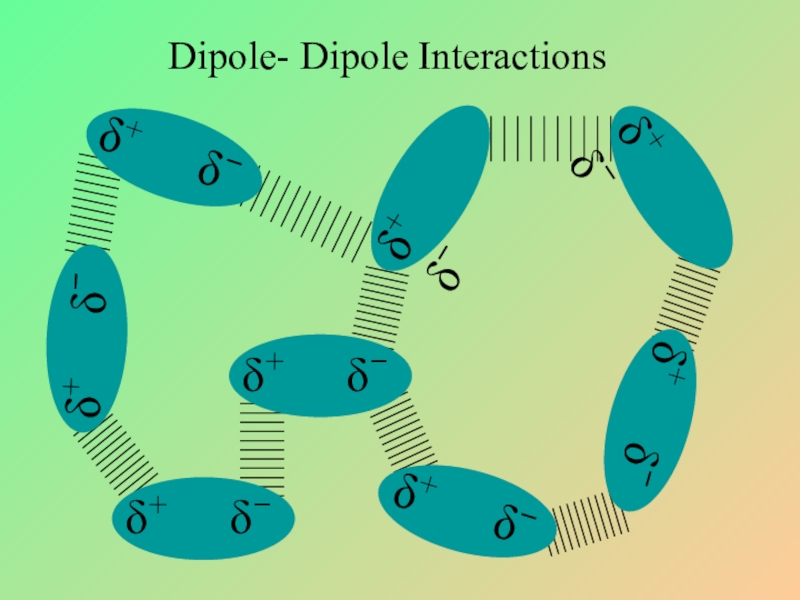
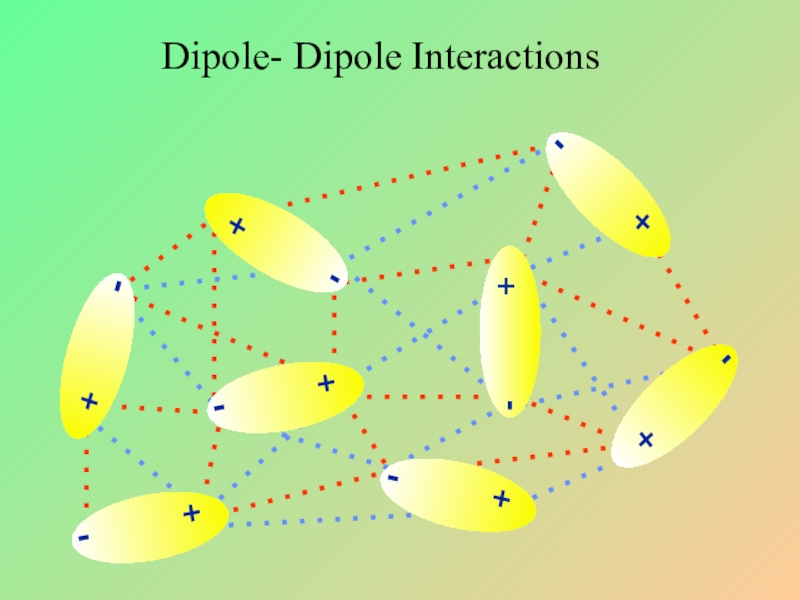
- 21. Dipole- Dipole Interactions
- 22. Слайд 22
- 23. Dipole- Dipole Interactions
- 24. VAN DER WAALS FORCESThe intermolecular forces between
- 25. Increasing number of atoms in a molecule
- 26. Слайд 26
- 27. Слайд 27
- 28. Formation of van der Waals forces. Molecules approaching each other gain a momentarily polar character.
- 29. Gecko, a kind of lizard, is able
- 30. HYDROGEN BONDSAn extra strong dipole-dipole attraction that
- 31. Hydrogen bonds between water molecules are stronger than dipole-dipole and van der Waals forces.
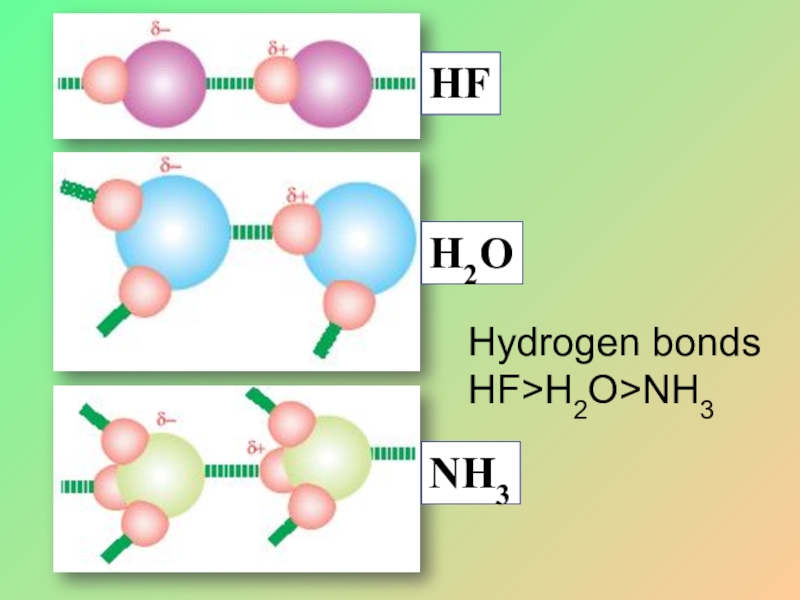
- 32. Hydrogen bonds HF>H2O>NH3
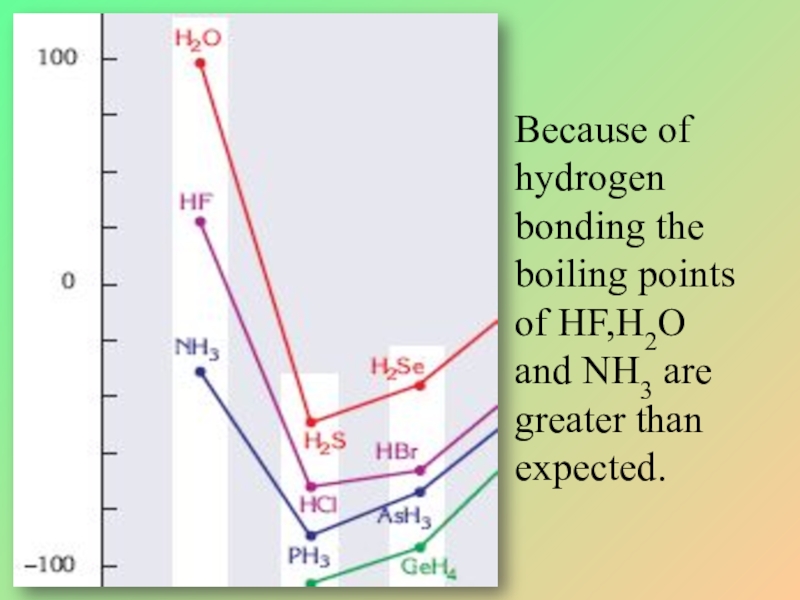
- 33. Because of hydrogenbonding the boiling points of HF,H2Oand NH3 are greater than expected.
- 34. Ionic bondhereIonic bondcovalent bondmetallic bondhydrogen bondJames
- 35. Слайд 35
- 36. Each of snowflakes is different from each
Слайд 2INDEX
INTRODUCTION
METALLIC BONDS
IONIC SOLIDS
NETWORK SOLIDS
DIPOLE–DIPOLE FORCES
VAN DER WAALS FORCES
HYDROGEN BONDS
Слайд 3INTRODUCTION
In the solid or liquid phase molecules are very close to
We have already studied intramolecular bonds in molecules now, we will examine intermolecular forces of attraction in liquids and solids.
Слайд 4METALLIC BONDS
Metals are solid at room temperature except for mercury.
The
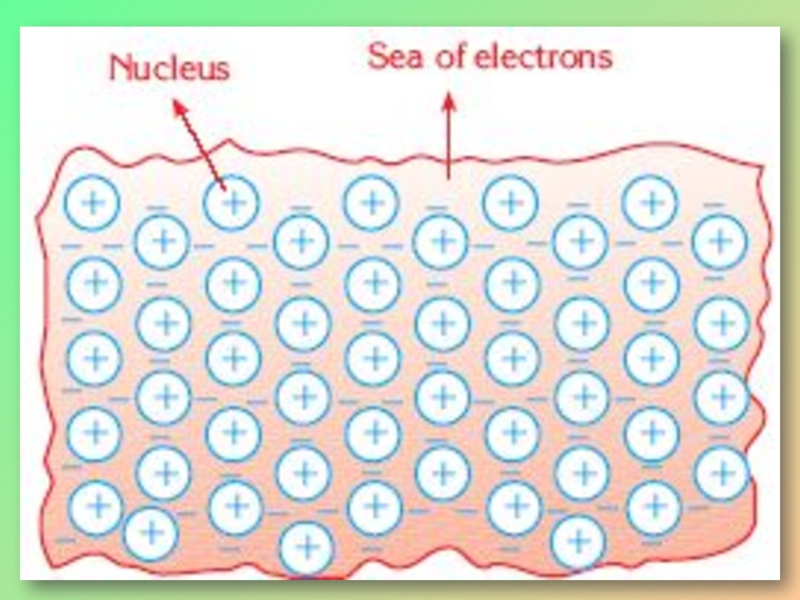
The valence electrons of metal atoms can easily move from the free orbitals of one atom to another and form an “electron sea”.
Слайд 5Attraction force between the negatively charged “sea of electrons” and the
This is called the metallic bond.
In a group, metallic bond strength generally decreases from up to down.
In a period, strength generally increases from left to right.
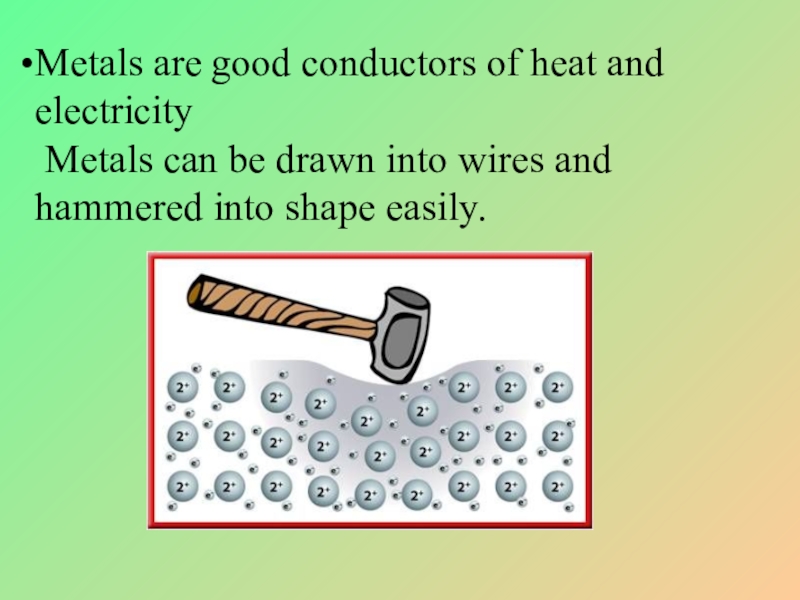
Слайд 7Metals are good conductors of heat and electricity
Metals can be
Слайд 12IONIC SOLIDS
When metal and nonmetal atoms come together, they form ionic
Electrostatic attraction occurs between
the positive and negative charges holding the ions together.
Metal ions are surrounded by nonmetal ions and nonmetal ions surrounded by metal ions.
Слайд 13.
Ionic solids do not have a molecular structure.
The melting and
Aqueous solution and molten state conduct electricity.
Don’t conduct electricity
Brittle, not ductile and can not be drawn into wires or hammered into plates.
Very stable
Слайд 14NETWORK SOLIDS
In some covalent substances, known as network solids, atoms are
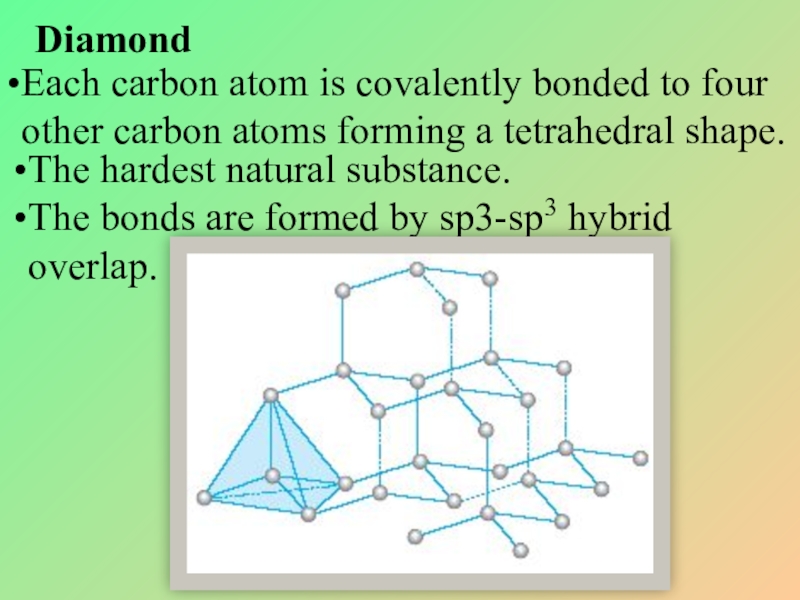
Слайд 15Diamond
Each carbon atom is covalently bonded to four other carbon atoms
The hardest natural substance.
The bonds are formed by sp3-sp3 hybrid overlap.
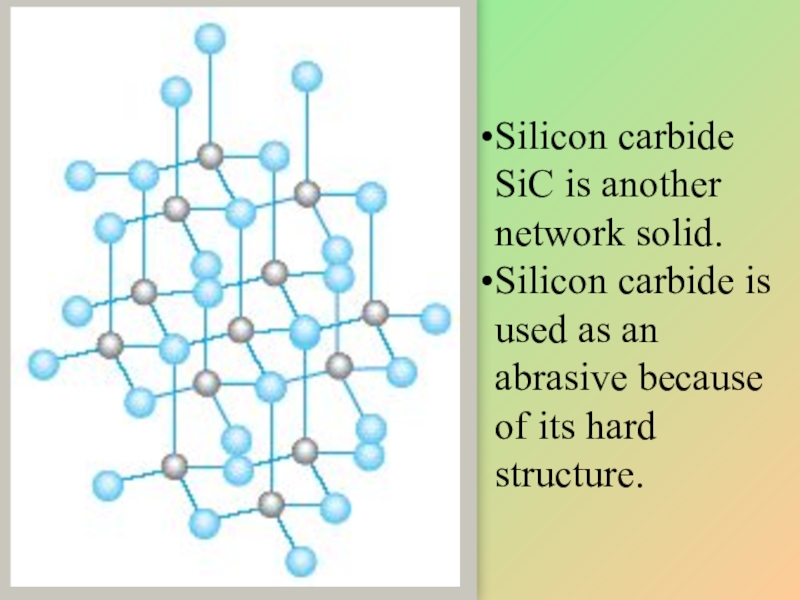
Слайд 16Silicon carbide SiC is another network solid.
Silicon carbide is used
Слайд 17Diamond and silicon carbide are nonconductors of electricity and have very
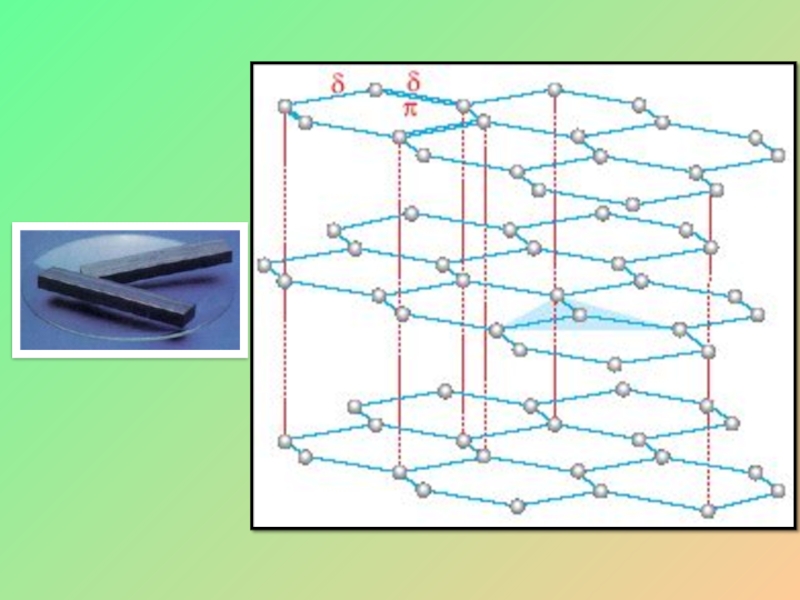
Слайд 18Graphite
Each carbon atom is bonded to three other carbon atoms with
The bonding involves sp2 – sp2 hybrid overlap.
Because of weak bonding between the layers, the layers can slide over each other.
Conduct electricity.
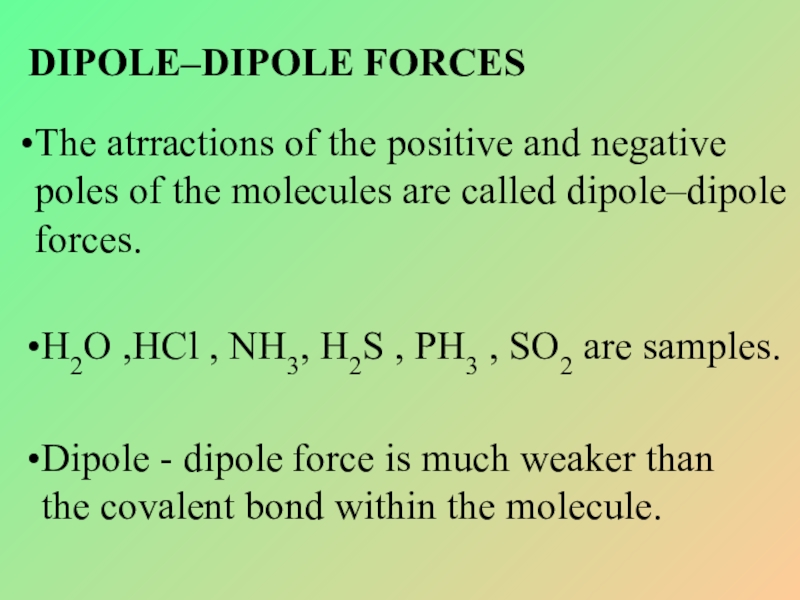
Слайд 20DIPOLE–DIPOLE FORCES
The atrractions of the positive and negative poles of the
H2O ,HCl , NH3, H2S , PH3 , SO2 are samples.
Dipole - dipole force is much weaker than the covalent bond within the molecule.
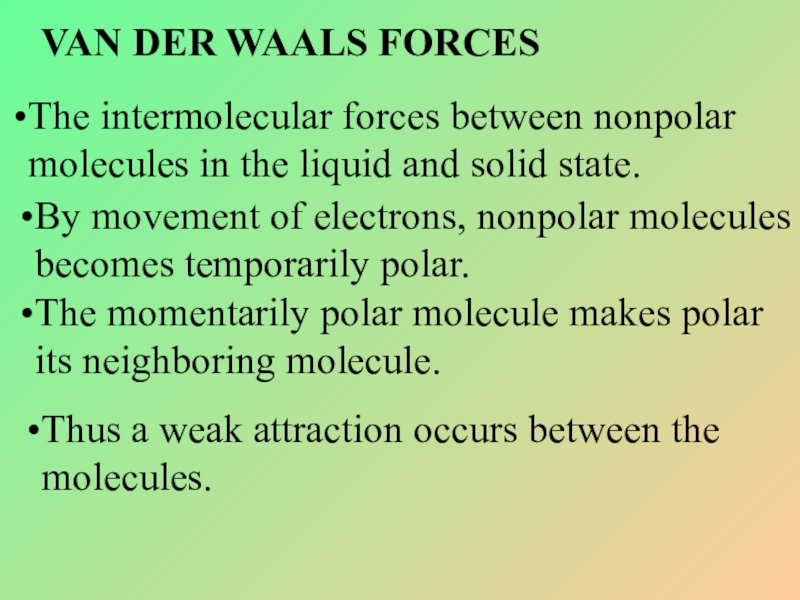
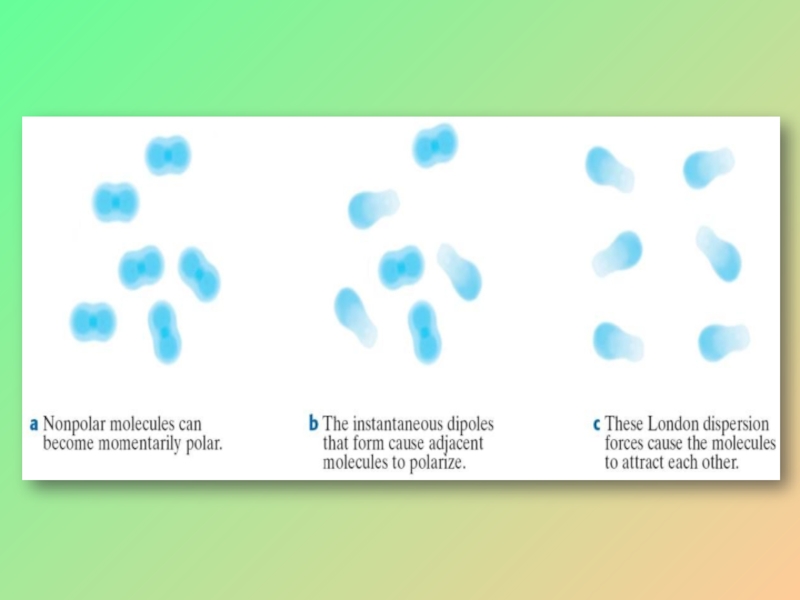
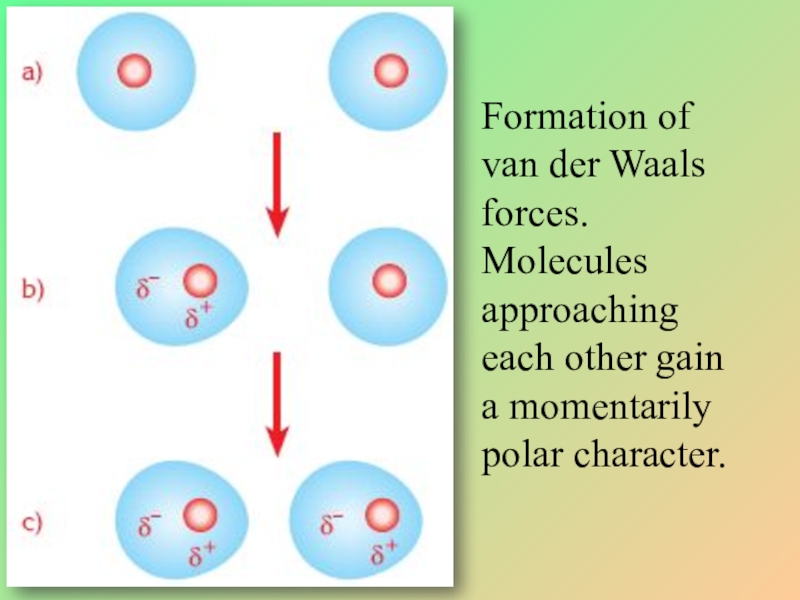
Слайд 24VAN DER WAALS FORCES
The intermolecular forces between nonpolar molecules in the
By movement of electrons, nonpolar molecules becomes temporarily polar.
The momentarily polar molecule makes polar its neighboring molecule.
Thus a weak attraction occurs between the molecules.
Слайд 25Increasing number of atoms in a molecule increases the van der
Van der Waals forces are stronger between molecules with high molecular masses.
For small molecules, the van der Waals force is weaker than dipole - dipole forces and hydrogen bonding.
Слайд 28Formation of van der Waals forces. Molecules approaching each other gain
Слайд 29Gecko, a kind of lizard, is able to move freely on
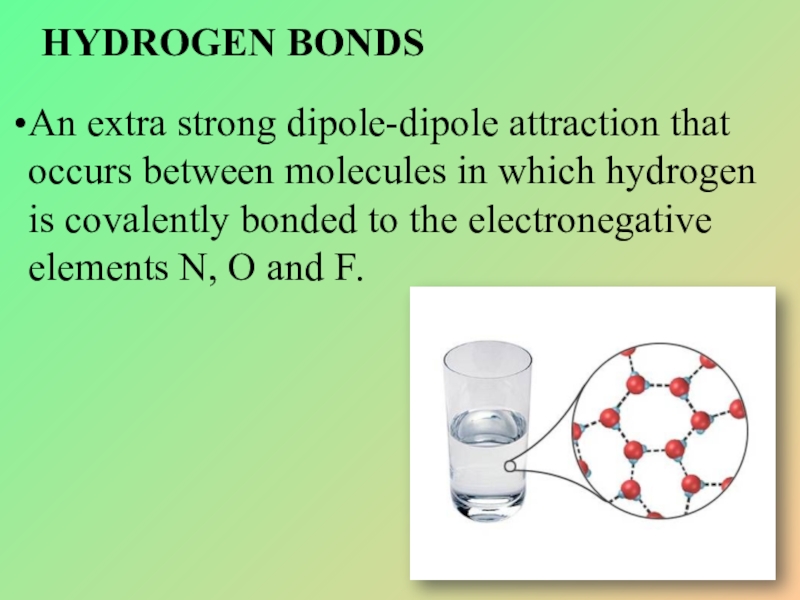
Слайд 30HYDROGEN BONDS
An extra strong dipole-dipole attraction that occurs between molecules in
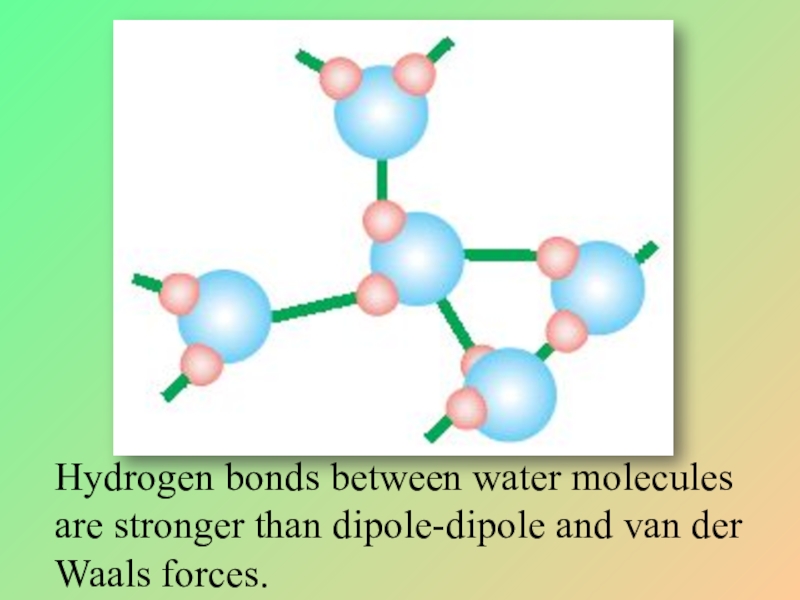
Слайд 31Hydrogen bonds between water molecules are stronger than dipole-dipole and van
Слайд 34Ionic bond
here
Ionic bond
covalent bond
metallic bond
hydrogen bond
James
He! He ! He! absent