- Главная
- Разное
- Образование
- Спорт
- Естествознание
- Природоведение
- Религиоведение
- Французский язык
- Черчение
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, фоны, картинки для презентаций
- Экология
- Экономика
Презентация, доклад химия пәнінен (8 сынып)
Содержание
- 1. Презентация химия пәнінен (8 сынып)
- 2. Coloring in the Periodic Table
- 3. Homework According to the periodic trends in
- 4. Physical properties of METALS
- 5. Physical Properties of METALSMetals have shiny luster.
- 6. Physical Properties of METALSMetals are good conductors
- 7. Physical Properties of METALSMetals are malleable.
- 8. Physical Properties of METALSMetals are ductile.
- 9. Examples of NONMETALS Nonmetals may be solids,
- 10. Physical Properties of NONMETALSNonmetals have a dull luster. (They are not shiny!)Example: Phosphorus
- 11. Physical Properties of NONMETALS Nonmetals are insulators.
- 12. Physical Properties of NONMETALSNonmetals are soft (except for diamonds and brittle.Example: Sulfur
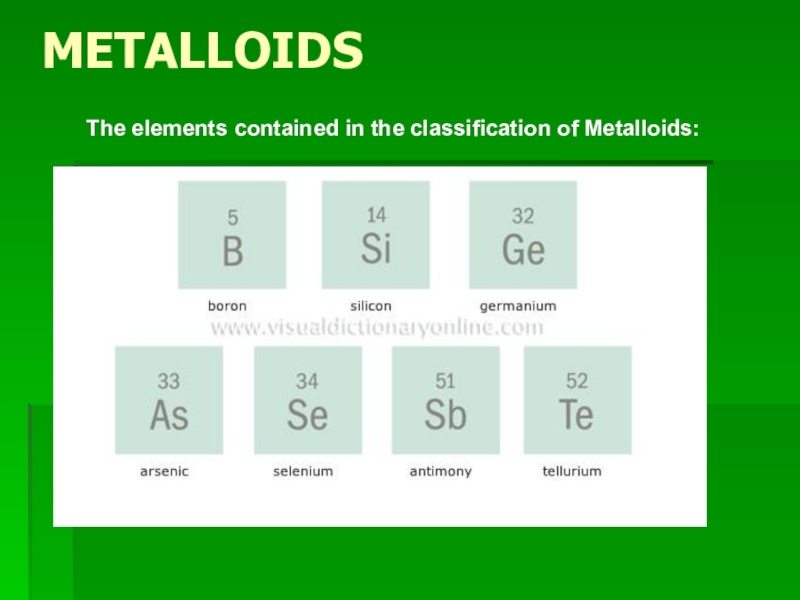
- 13. METALLOIDSThe elements contained in the classification of Metalloids:
- 14. METALLOIDSElements classified as Metalloids have physical properties
- 15. METALLOIDSSome metalloids are useful semiconductors, which are
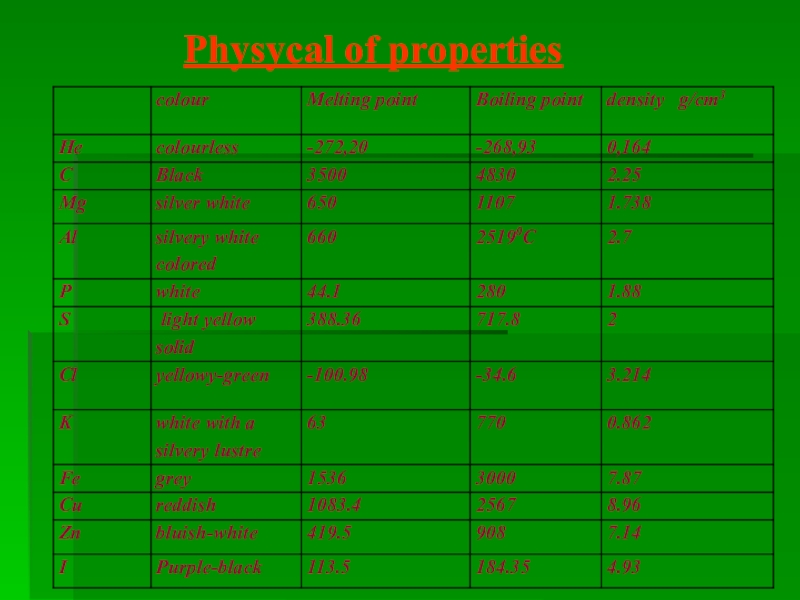
- 16. Physycal of properties
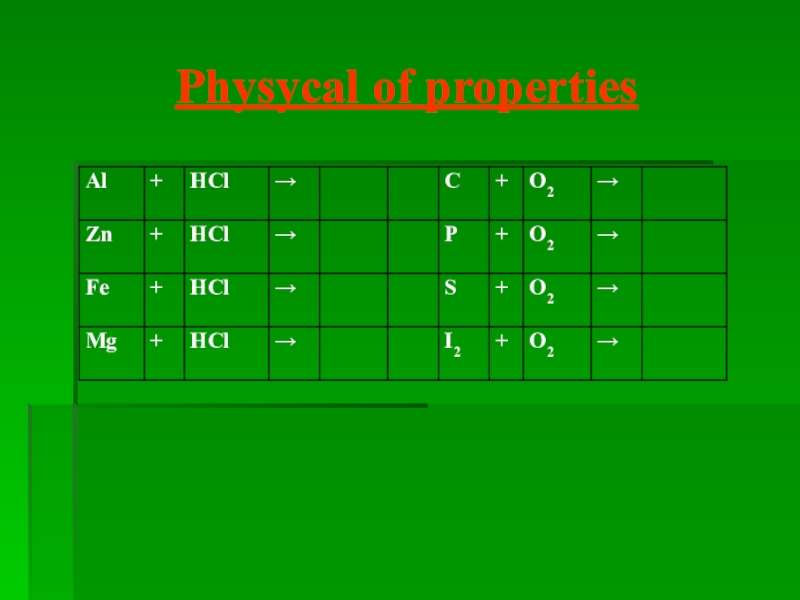
- 17. Physycal of properties
- 18. Сараланған тапсырмалар:-The ions 21X3+ and Y2- have
- 19. Homework For a neutral 3580Br atom:Find the
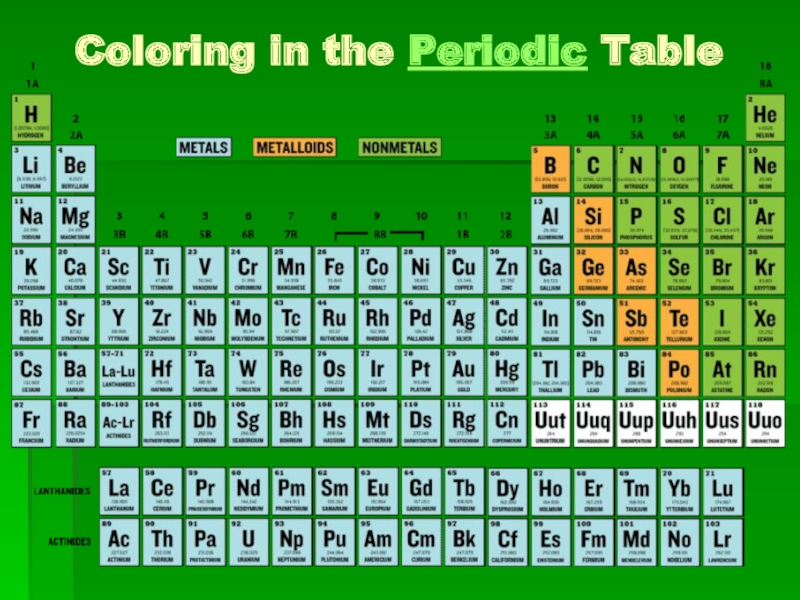
Coloring in the Periodic Table
Слайд 3Homework
According to the periodic trends in metallic properties, which element
have the greater atomic radius?
Sort the elements in order of increasing metallic properties: Ca, Mg, Sr, Be, Ba
Why is the electronegativity value of most noble gases zero?
A nonmetal has a smaller ionic radius compared with a metal of the same period. Do you agree? Why?
Have you ever heard that metals have a memory?
Lesson plan:
1. Elements: “Metals”, “Nonmetal”, “Matelloids”
2. Physical and chemical properties
3. Science in context, facts and terminology
4. Activity
Sort the elements in order of increasing metallic properties: Ca, Mg, Sr, Be, Ba
Why is the electronegativity value of most noble gases zero?
A nonmetal has a smaller ionic radius compared with a metal of the same period. Do you agree? Why?
Have you ever heard that metals have a memory?
Lesson plan:
1. Elements: “Metals”, “Nonmetal”, “Matelloids”
2. Physical and chemical properties
3. Science in context, facts and terminology
4. Activity
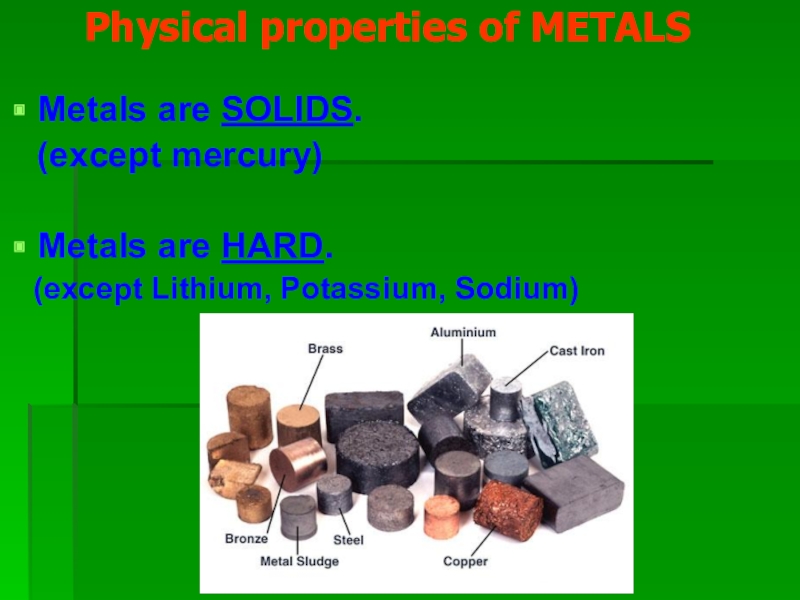
Слайд 4 Physical properties of METALS
Metals are SOLIDS.
(except mercury)
Metals are HARD.
(except Lithium, Potassium, Sodium)
Metals are HARD.
(except Lithium, Potassium, Sodium)
Слайд 5Physical Properties of METALS
Metals have shiny luster. (or metallic luster)
LUSTER – the way an object’s surface reflects light
Слайд 6Physical Properties of METALS
Metals are good conductors of electricity.
Copper, silver,
and gold are good electrical conductors. In a conductor, electric current can flow freely. Since metals have free electrons, they can carry a charge easily.
Copper Wiring
Слайд 7Physical Properties of METALS
Metals are malleable.
Malleable or Malleability -
metals ability to be shaped or formed as by hammering or pressure; can be beaten into thin sheets
Aluminum is malleable.
Aluminum is malleable.
Слайд 8Physical Properties of METALS
Metals are ductile.
Ductility or ductile –
can be drawn into a wire
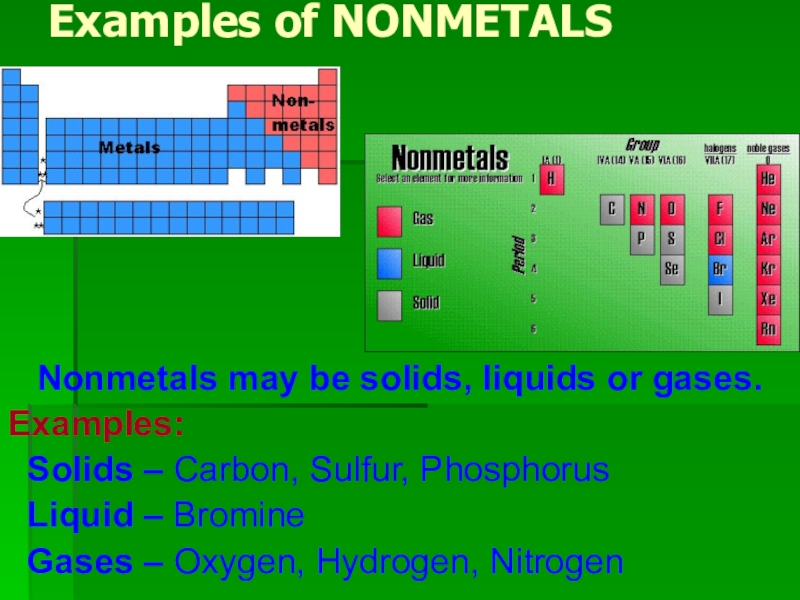
Слайд 9Examples of NONMETALS
Nonmetals may be solids, liquids or gases.
Examples:
Solids – Carbon, Sulfur, Phosphorus
Liquid – Bromine
Gases – Oxygen, Hydrogen, Nitrogen
Liquid – Bromine
Gases – Oxygen, Hydrogen, Nitrogen
Слайд 10Physical Properties of NONMETALS
Nonmetals have a dull luster.
(They are not
shiny!)
Example: Phosphorus
Example: Phosphorus
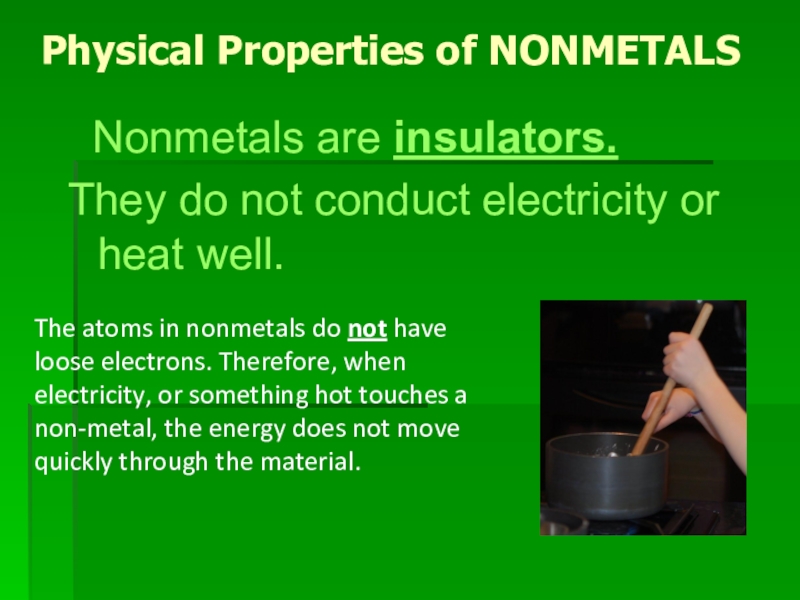
Слайд 11Physical Properties of NONMETALS
Nonmetals are insulators.
They do not conduct
electricity or heat well.
The atoms in nonmetals do not have loose electrons. Therefore, when electricity, or something hot touches a non-metal, the energy does not move quickly through the material.
Слайд 12Physical Properties of NONMETALS
Nonmetals are soft (except for diamonds and brittle.
Example:
Sulfur
Слайд 14METALLOIDS
Elements classified as Metalloids have physical properties of both metals and
non-metals.
Some are shiny, some are dull, they are somewhat malleable and ductile, and can conduct heat and electricity at a lesser level than metals.
Some are shiny, some are dull, they are somewhat malleable and ductile, and can conduct heat and electricity at a lesser level than metals.
BORON
SILICON
ARSENIC
Слайд 15METALLOIDS
Some metalloids are useful semiconductors, which are used in electronics (radio,
computers, telephones, etc.)
They are useful because they conduct just the right amount of electricity or heat.
They are useful because they conduct just the right amount of electricity or heat.
Слайд 18Сараланған тапсырмалар:
-The ions 21X3+ and Y2- have the same electron configuration.
Find the place of Y in the Periodic Table.
-Find the numbers of valence electrons fo the elements magnesium (12Mg), oxygen (8O), silicon (14Si) and zink (30Zn)
-The atomic mass number of X is 70, and the number of neutrons of X is 39. What are the group and period numbers of element X in the Periodic Table?
-An Z2- ion has 18 electrons. What are the group and period number of the Z atom in the Periodic Table?
-Find the numbers of valence electrons fo the elements magnesium (12Mg), oxygen (8O), silicon (14Si) and zink (30Zn)
-The atomic mass number of X is 70, and the number of neutrons of X is 39. What are the group and period numbers of element X in the Periodic Table?
-An Z2- ion has 18 electrons. What are the group and period number of the Z atom in the Periodic Table?
Слайд 19Homework
For a neutral 3580Br atom:
Find the number of neutrons and
protons in the nucleus.
How many s electrons does it have in total?
How many electrons does it have in 4p orbitals?
Which type of orbital has the more electron than other?
How many s electrons does it have in total?
How many electrons does it have in 4p orbitals?
Which type of orbital has the more electron than other?