қабаттарда орналасуының схемасын салу
8.1.3.3 электрондар атомда ядродан арақашықтықтары артып келе жатқан орбитальдарда біртіндеп орналасатындығын түсіну
- Главная
- Разное
- Образование
- Спорт
- Естествознание
- Природоведение
- Религиоведение
- Французский язык
- Черчение
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, фоны, картинки для презентаций
- Экология
- Экономика
Презентация, доклад на тему Химия пәнінен Атом құрылысы тақырыбында Презентация жұмысы
Содержание
- 1. Химия пәнінен Атом құрылысы тақырыбында Презентация жұмысы
- 2. Lesson objectives: Сабақ мақсаты8.1.3.1 бірінші 20 элементтің
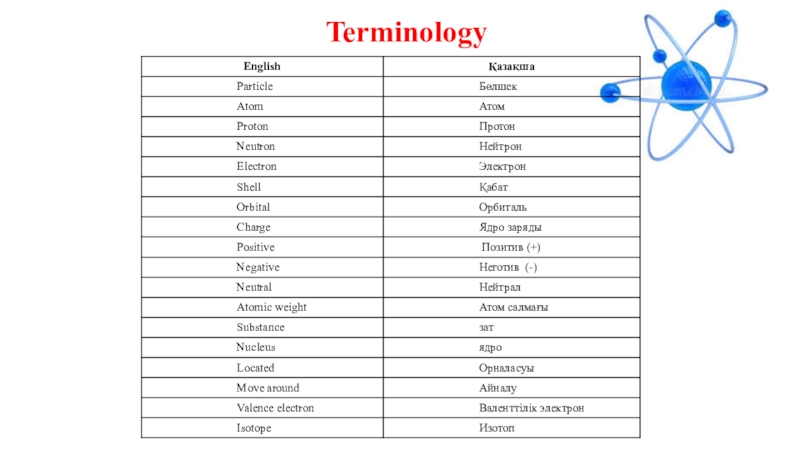
- 3. Terminology
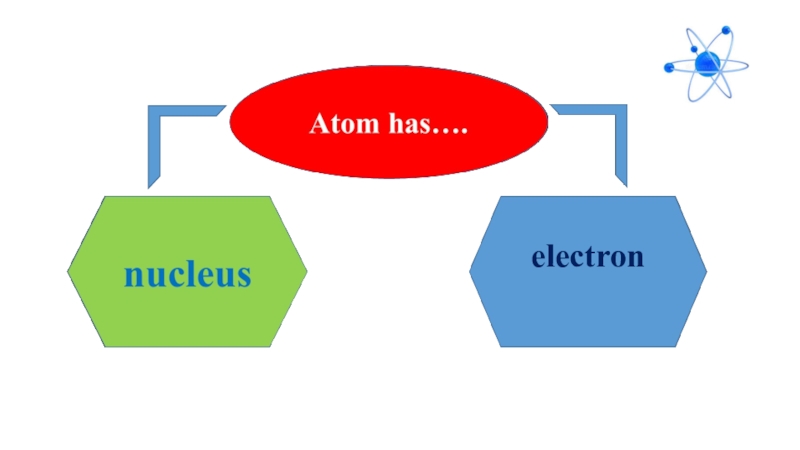
- 4. electron
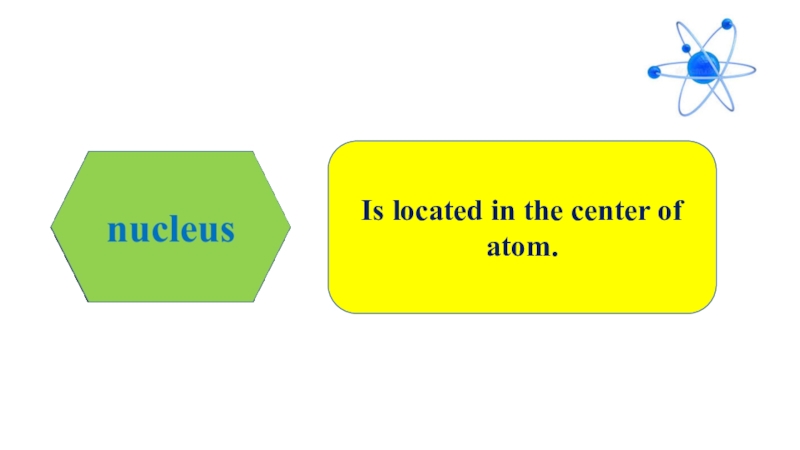
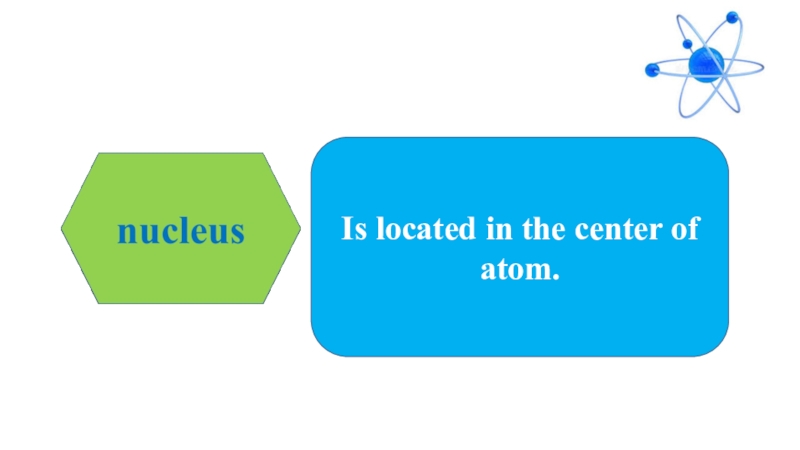
- 5. Is located in the center of atom.
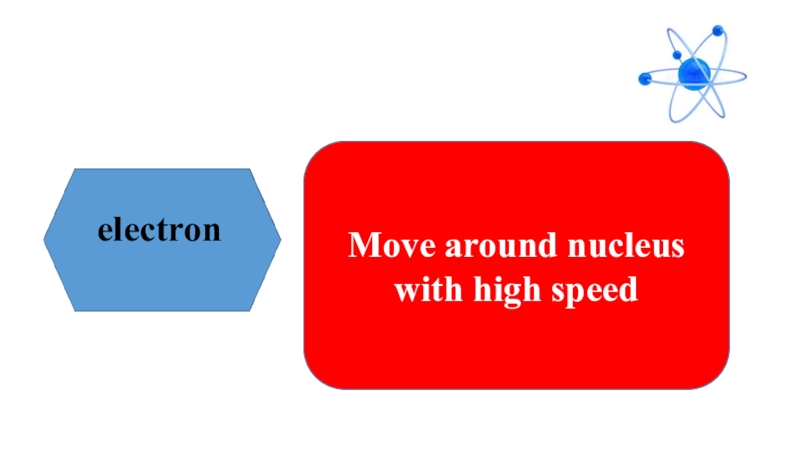
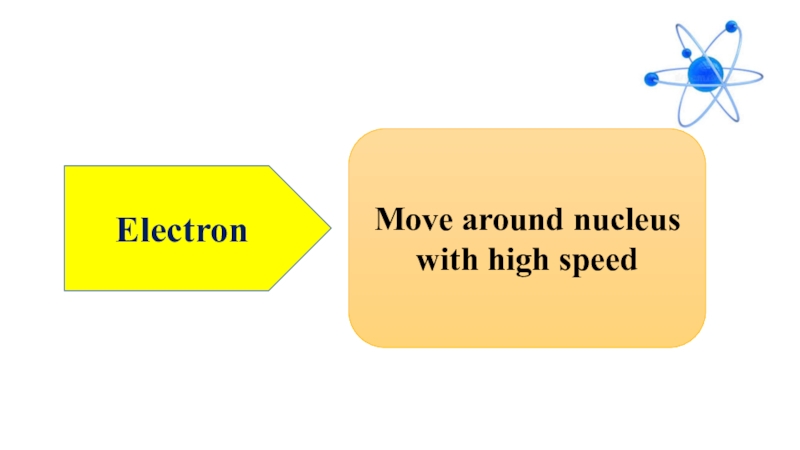
- 6. electronMove around nucleus with high speed
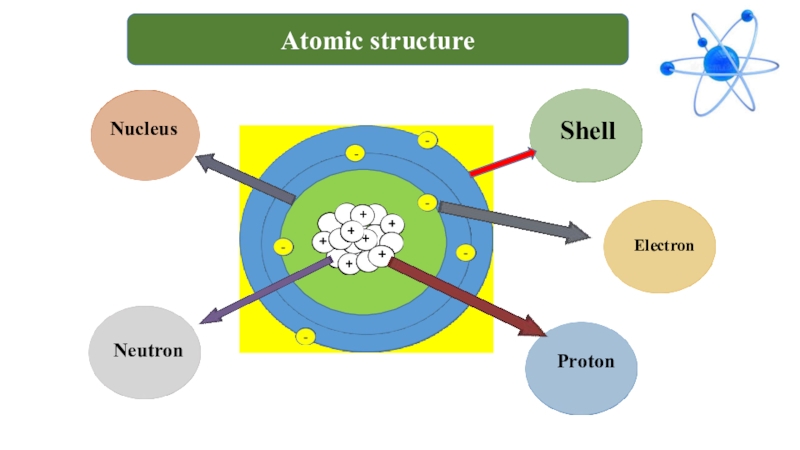
- 7. +-ShellElectronProtonNeutronNucleusAtomic structure
- 8. Is located in the center of atom.
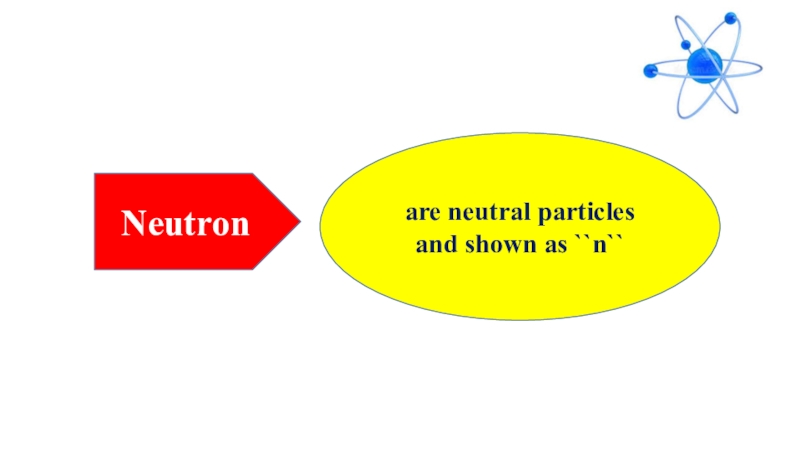
- 9. Neutronare neutral particles and shown as ``n``
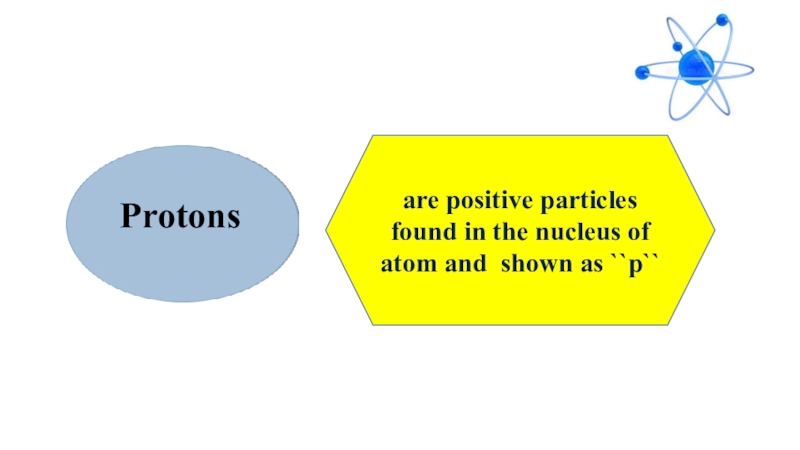
- 10. Protonsare positive particles found in the nucleus of atom and shown as ``p``
- 11. ElectronMove around nucleus with high speed
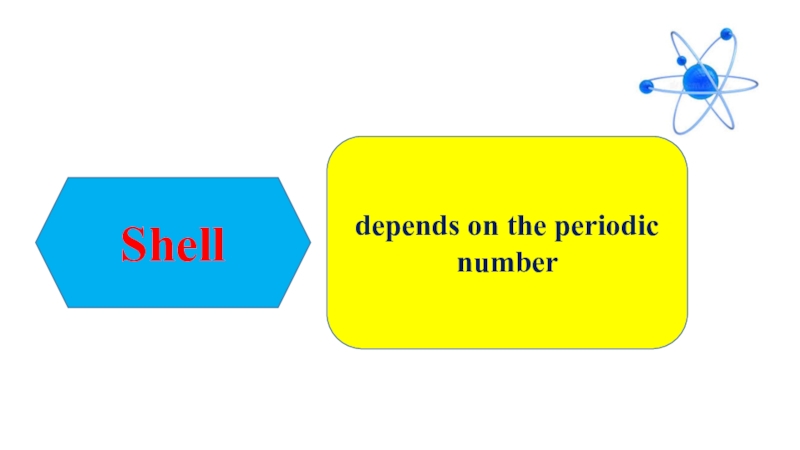
- 12. Shelldepends on the periodic number
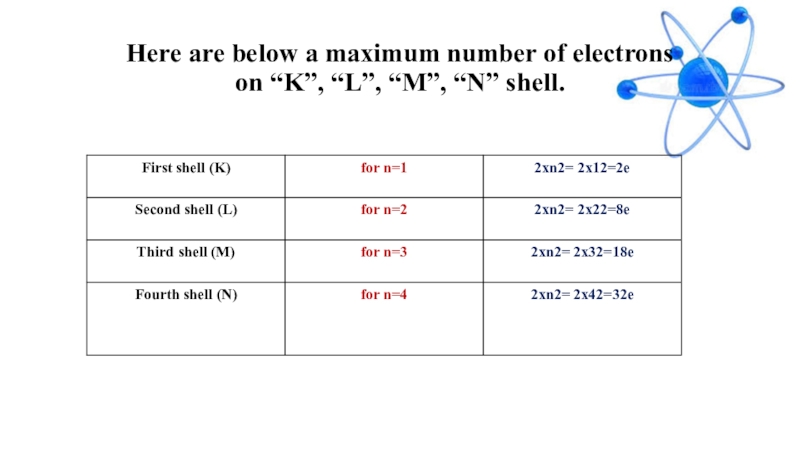
- 13. Here are below a maximum number of electrons on “K”, “L”, “M”, “N” shell.
- 14. Слайд 14
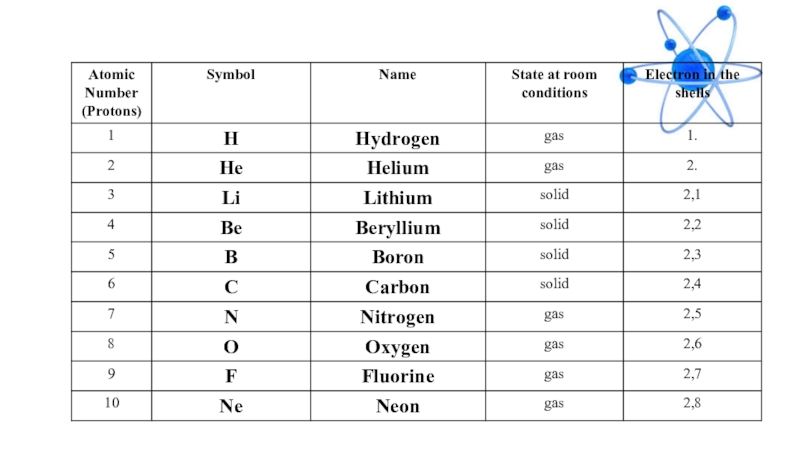
- 15. General description of element
- 16. Слайд 16
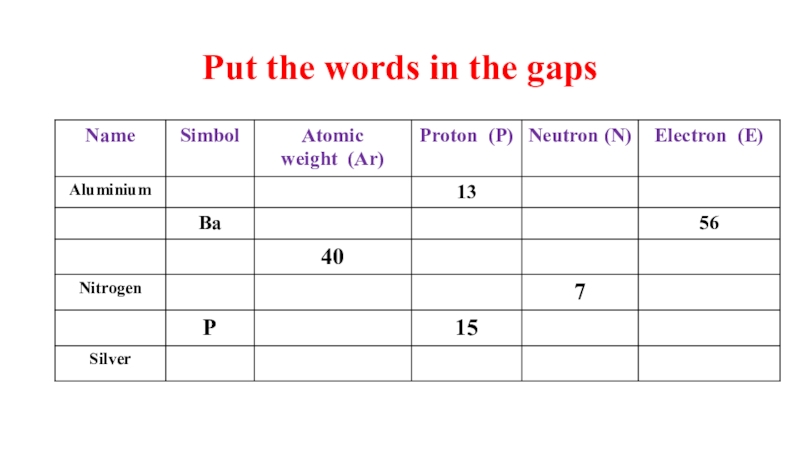
- 17. Put the words in the gaps
- 18. Nucleus shell, atom neutral, negative electrons orbital,
- 19. Nucleus shell, atom neutral, negative electrons orbital,
- 20. Complete the sentences and pronounce themAtom is
- 21. Complete the sentences and pronounce themAtom is
- 22. Постер қорғау
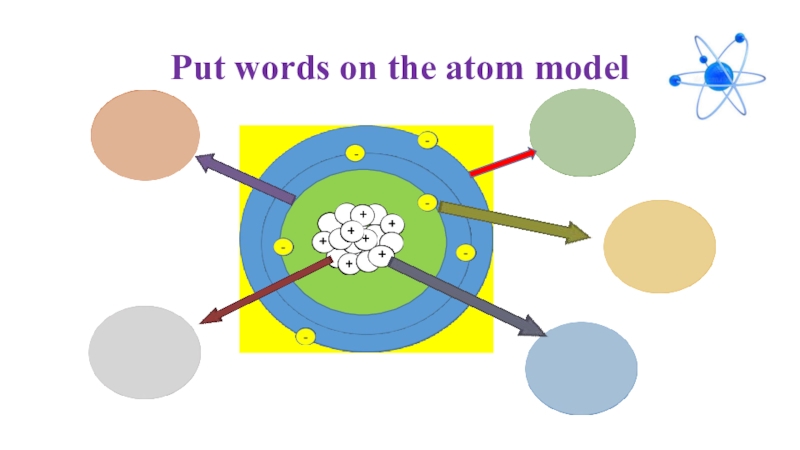
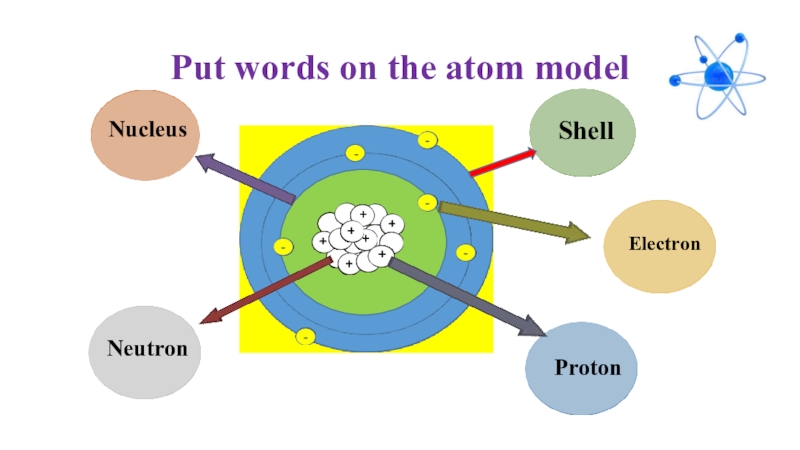
- 23. Put words on the atom model+-
- 24. Put words on the atom model+-ShellElectronProtonNeutronNucleus
- 25. Work in pairs. Ask the following question
Lesson objectives: Сабақ мақсаты8.1.3.1 бірінші 20 элементтің электрондар санын анықтау 8.1.3.2 электрондардың қабаттарда орналасуының схемасын салу 8.1.3.3 электрондар атомда ядродан арақашықтықтары артып келе жатқан орбитальдарда біртіндеп орналасатындығын түсіну
Слайд 2Lesson objectives:
Сабақ мақсаты
8.1.3.1 бірінші 20 элементтің электрондар санын анықтау
8.1.3.2 электрондардың
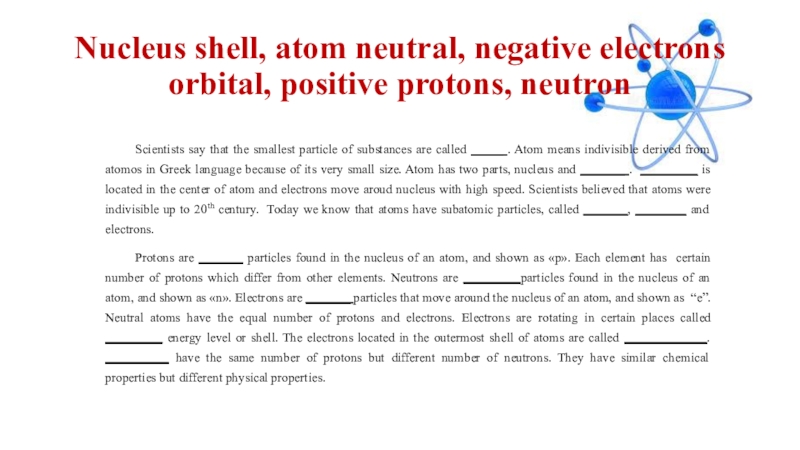
Слайд 18Nucleus shell, atom neutral, negative electrons orbital, positive protons, neutron
Scientists say
that the smallest particle of substances are called _____ . Atom means indivisible derived from atomos in Greek language because of its very small size. Atom has two parts, nucleus and _______ . _________ is located in the center of atom and electrons move aroud nucleus with high speed. Scientists believed that atoms were indivisible up to 20th century. Today we know that atoms have subatomic particles, called _______, ________ and electrons.
Protons are _______ particles found in the nucleus of an atom, and shown as «p». Each element has certain number of protons which differ from other elements. Neutrons are _________particles found in the nucleus of an atom, and shown as «n». Electrons are _______ particles that move around the nucleus of an atom, and shown as “e”. Neutral atoms have the equal number of protons and electrons. Electrons are rotating in certain places called _________ energy level or shell. The electrons located in the outermost shell of atoms are called _____________. __________ have the same number of protons but different number of neutrons. They have similar chemical properties but different physical properties.
Protons are _______ particles found in the nucleus of an atom, and shown as «p». Each element has certain number of protons which differ from other elements. Neutrons are _________particles found in the nucleus of an atom, and shown as «n». Electrons are _______ particles that move around the nucleus of an atom, and shown as “e”. Neutral atoms have the equal number of protons and electrons. Electrons are rotating in certain places called _________ energy level or shell. The electrons located in the outermost shell of atoms are called _____________. __________ have the same number of protons but different number of neutrons. They have similar chemical properties but different physical properties.
Слайд 19Nucleus shell, atom neutral, negative electrons orbital, positive protons, neutron
Scientists say
that the smallest particle of substances are called atom. Atom means indivisible derived from atomos in Greek language because of its very small size. Atom has two parts, nucleus and electron. Electron is located in the center of atom and electrons move aroud nucleus with high speed. Scientists believed that atoms were indivisible up to 20th century. Today we know that atoms have subatomic particles, called proton, neutron and electrons.
Protons are positive particles found in the nucleus of an atom, and shown as «p». Each element has certain number of protons which differ from other elements. Neutrons are neutral particles found in the nucleus of an atom, and shown as «n». Electrons are negative particles that move around the nucleus of an atom, and shown as “e”. Neutral atoms have the equal number of protons and electrons. Electrons are rotating in certain places called negative energy level or shell. The electrons located in the outermost shell of atoms are called valence electron . Isotopes have the same number of protons but different number of neutrons. They have similar chemical properties but different physical properties.
Protons are positive particles found in the nucleus of an atom, and shown as «p». Each element has certain number of protons which differ from other elements. Neutrons are neutral particles found in the nucleus of an atom, and shown as «n». Electrons are negative particles that move around the nucleus of an atom, and shown as “e”. Neutral atoms have the equal number of protons and electrons. Electrons are rotating in certain places called negative energy level or shell. The electrons located in the outermost shell of atoms are called valence electron . Isotopes have the same number of protons but different number of neutrons. They have similar chemical properties but different physical properties.
Слайд 20Complete the sentences and pronounce them
Atom is _____________
From Greek language atomos
means _____________
Atom has two parts: ______ and _______
Protons are _____________ and shown as `` p``
Neutrons are _________ and shown as ``n``
Electrons are _________ and shown as ``e``
Isotopes have ___________________ .
Atom has two parts: ______ and _______
Protons are _____________ and shown as `` p``
Neutrons are _________ and shown as ``n``
Electrons are _________ and shown as ``e``
Isotopes have ___________________ .
Слайд 21Complete the sentences and pronounce them
Atom is the smallest particle of
substances
From Greek language atomos means very small size
Atom has two parts: nucleus and electron
Protons are positive particles found in the nucleus of atom and shown as `` p``
Neutrons are neutral particles and shown as ``n``
Electrons are negative particles than move around the nucleus of on atom and shown and shown as ``e``
Isotopes have the some number of protons but different number of neutrons .
From Greek language atomos means very small size
Atom has two parts: nucleus and electron
Protons are positive particles found in the nucleus of atom and shown as `` p``
Neutrons are neutral particles and shown as ``n``
Electrons are negative particles than move around the nucleus of on atom and shown and shown as ``e``
Isotopes have the some number of protons but different number of neutrons .
Слайд 25Work in pairs. Ask the following question to a partner
What is
atom?
What is the meaning of atomos from Greek language?
What are the two parts that atom has?
What is definition of protons?
What is definition of neutrons?
What is definition of electrons?
What is definition of isotope?
What is the meaning of atomos from Greek language?
What are the two parts that atom has?
What is definition of protons?
What is definition of neutrons?
What is definition of electrons?
What is definition of isotope?