- Главная
- Разное
- Образование
- Спорт
- Естествознание
- Природоведение
- Религиоведение
- Французский язык
- Черчение
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- Детские презентации
- Информатика
- История
- Литература
- Математика
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, фоны, картинки для презентаций
- Экология
- Экономика
Презентация, доклад Билингвальный урок Клеточный метоболизм
Содержание
- 1. Презентация Билингвальный урок Клеточный метоболизм
- 2. Cellular MetabolismCellular metabolism refers to all of the chemical processes that occur inside living cells.
- 3. EnergyEnergy can exist in two states:Kinetic energy
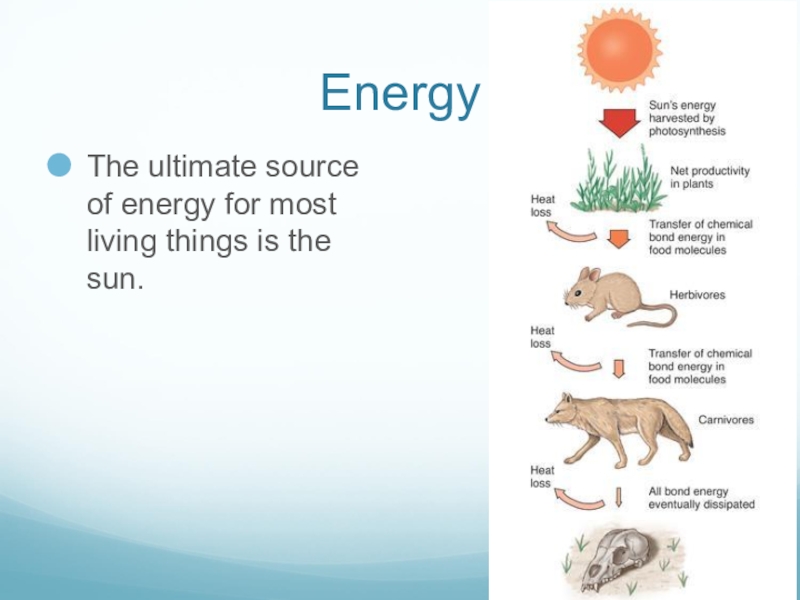
- 4. EnergyThe ultimate source of energy for most living things is the sun.
- 5. Laws of ThermodynamicsFirst law of thermodynamics –
- 6. Free EnergyFree energy – the energy available
- 7. EnzymesBonds must be destabilized before any reaction
- 8. EnzymesCatalysts are chemical substances that speed up
- 9. EnzymesEnzymes reduce the amount of activation energy
- 10. EnzymesEnzymes may be pure proteins or proteins
- 11. Enzyme FunctionAn enzyme works by binding with
- 12. Enzyme SpecificityEnzymes are highly specific.There is an
- 13. Enzyme-Catalyzed ReactionsEnzyme-catalyzed reactions are reversible.Indicated by double
- 14. Importance of ATPEndergonic reactions require energy to
- 15. Importance of ATPATP consists of adenosine (adenine
- 16. Importance of ATPPhosphates have negative charges.Takes lots
- 17. Importance of ATPA coupled reaction is a
- 18. Oxidation – Reduction - RedoxAn atom that
- 19. Redox ReactionsRedox reactions always occur in pairs.One
- 20. Cellular RespirationCellular respiration – the oxidation of
- 21. Cellular RespirationAerobic versus Anaerobic MetabolismHeterotrophsAerobes: Use molecular
- 22. Cellular RespirationWhen oxygen acts as the final
- 23. Aerobic RespirationIn aerobic respiration, ATP forms as
- 24. Cellular Respiration - 3 StagesFood is digested
- 25. Anaerobic RespirationAnaerobic respiration occurs in the absence
- 26. GlycolysisGlycolysis – the first stage in cellular
- 27. GlycolysisUphill portion primes the fuel with phosphates.Uses
- 28. GlycolysisSummary of the enzymatically catalyzed reactions in
- 29. Harvesting Electrons form Chemical BondsWhen oxygen is
- 30. Producing Acetyl-CoAThe 3-carbon pyruvate loses a carbon
- 31. The Krebs CycleThe Krebs cycle is the
- 32. Слайд 32
- 33. The Krebs CycleEach glucose provides 2 pyruvates,
- 34. The Krebs CycleAcetyl unit + 3 NAD+
- 35. Using Electrons to Make ATPNADH & FADH2
- 36. Building an Electrochemical GradientIn eukaryotes, aerobic metabolism
- 37. Producing ATP- ChemiosmosisA strong gradient with many
- 38. Слайд 38
- 39. Electron Transport Reviewhttp://www.youtube.com/watch?v=kN5MtqAB_Yc&list=FL9N_Px072WuVorSwDfqf-9w&index=2&feature=plpp
- 40. Review of Cellular Respiration1 ATP generated for
- 41. Glucose + 2 ATP + 36 ADP
- 42. FermentationIn the absence of oxygen, the end-product
- 43. Fermentation – 2 TypesAnimals add extracted electrons
- 44. Слайд 44
- 45. Metabolism of LipidsTriglycerides are broken down into
- 46. Metabolism of ProteinsProteins digested in the gut
- 47. Metabolism of ProteinsAmmonia is highly toxic, but
- 48. Regulating Cellular RespirationRate of cellular respiration slows
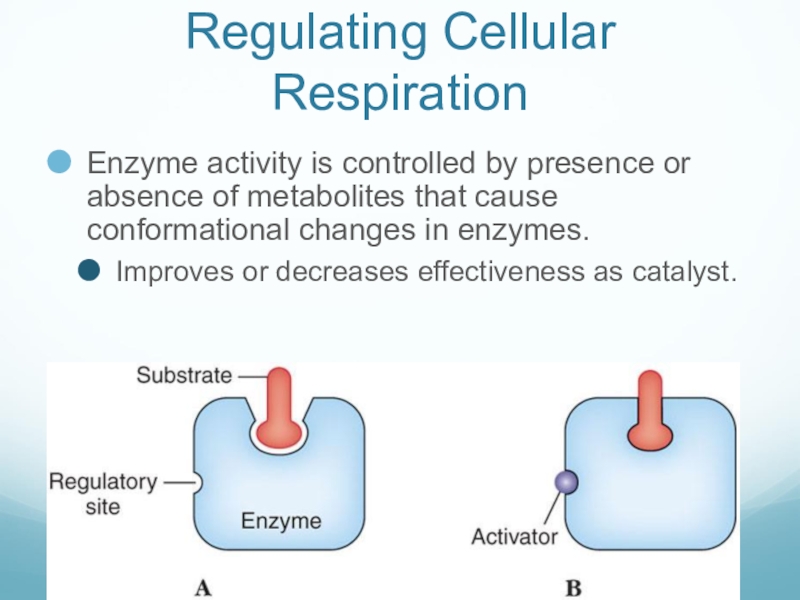
- 49. Regulating Cellular RespirationEnzyme activity is controlled by
Cellular MetabolismCellular metabolism refers to all of the chemical processes that occur inside living cells.
Слайд 2Cellular Metabolism
Cellular metabolism refers to all of the chemical processes that
occur inside living cells.
Слайд 3Energy
Energy can exist in two states:
Kinetic energy – energy of motion.
Potential
energy – stored energy.
Chemical energy – potential energy stored in bonds, released when bonds are broken.
Energy can be transformed form one state to another.
Chemical energy – potential energy stored in bonds, released when bonds are broken.
Energy can be transformed form one state to another.
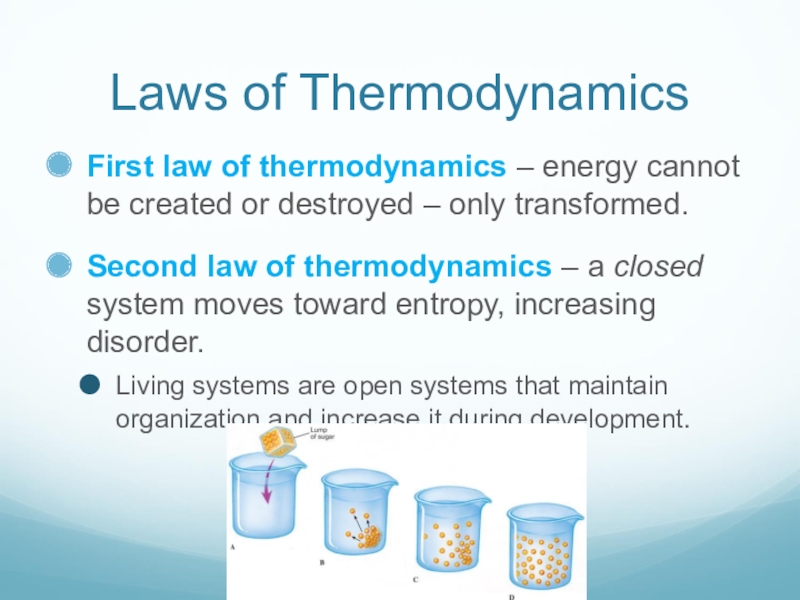
Слайд 5Laws of Thermodynamics
First law of thermodynamics – energy cannot be created
or destroyed – only transformed.
Second law of thermodynamics – a closed system moves toward entropy, increasing disorder.
Living systems are open systems that maintain organization and increase it during development.
Second law of thermodynamics – a closed system moves toward entropy, increasing disorder.
Living systems are open systems that maintain organization and increase it during development.
Слайд 6Free Energy
Free energy – the energy available for doing work.
Most chemical
reactions release free energy – they are exergonic.
Downhill
Some reactions require the input of free energy – they are endergonic.
Uphill
Downhill
Some reactions require the input of free energy – they are endergonic.
Uphill
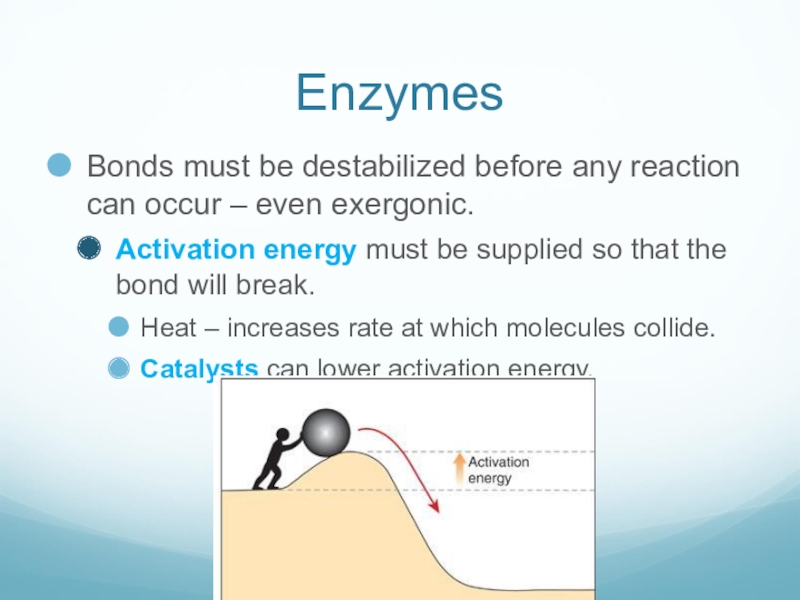
Слайд 7Enzymes
Bonds must be destabilized before any reaction can occur – even
exergonic.
Activation energy must be supplied so that the bond will break.
Heat – increases rate at which molecules collide.
Catalysts can lower activation energy.
Activation energy must be supplied so that the bond will break.
Heat – increases rate at which molecules collide.
Catalysts can lower activation energy.
Слайд 8Enzymes
Catalysts are chemical substances that speed up a reaction without affecting
the products.
Catalysts are not used up or changed in any way during the reaction.
Enzymes are important catalysts in living organisms.
Catalysts are not used up or changed in any way during the reaction.
Enzymes are important catalysts in living organisms.
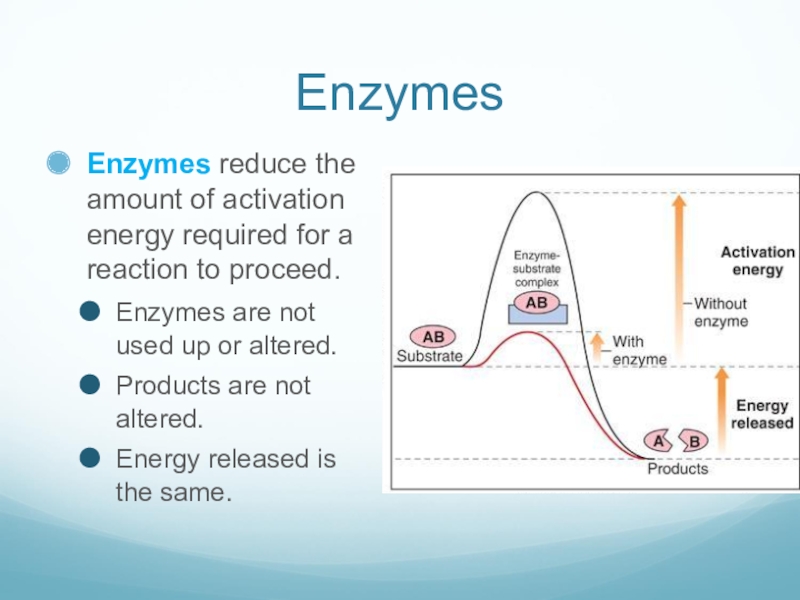
Слайд 9Enzymes
Enzymes reduce the amount of activation energy required for a reaction
to proceed.
Enzymes are not used up or altered.
Products are not altered.
Energy released is the same.
Enzymes are not used up or altered.
Products are not altered.
Energy released is the same.
Слайд 10Enzymes
Enzymes may be pure proteins or proteins plus cofactors such as
metallic ions or coenzymes, organic group that contain groups derived from vitamins.
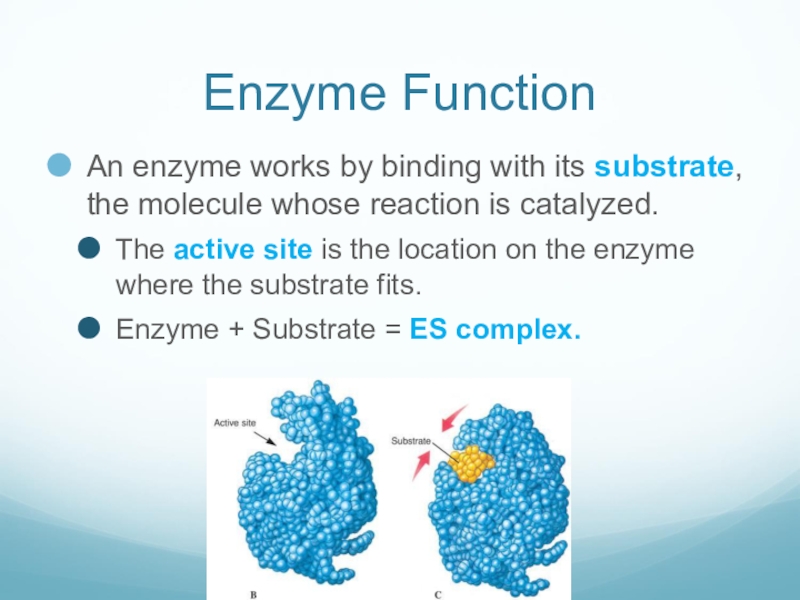
Слайд 11Enzyme Function
An enzyme works by binding with its substrate, the molecule
whose reaction is catalyzed.
The active site is the location on the enzyme where the substrate fits.
Enzyme + Substrate = ES complex.
The active site is the location on the enzyme where the substrate fits.
Enzyme + Substrate = ES complex.
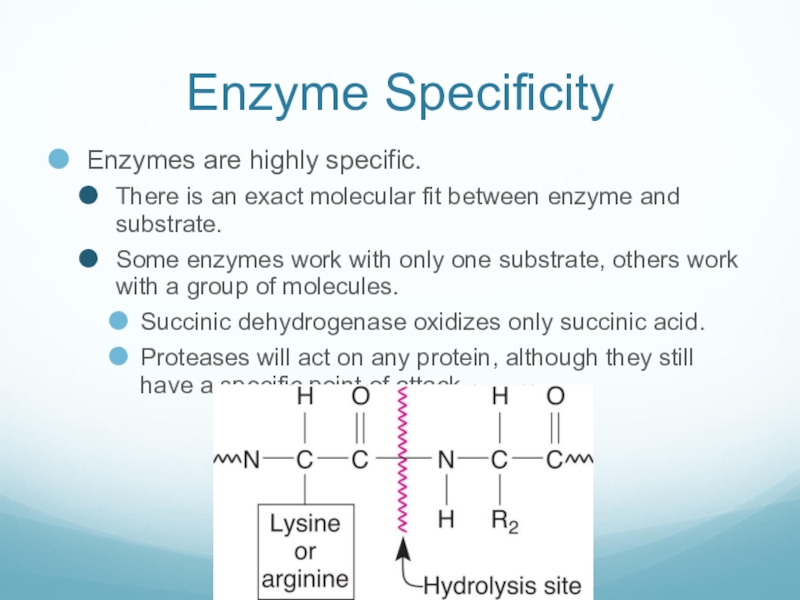
Слайд 12Enzyme Specificity
Enzymes are highly specific.
There is an exact molecular fit between
enzyme and substrate.
Some enzymes work with only one substrate, others work with a group of molecules.
Succinic dehydrogenase oxidizes only succinic acid.
Proteases will act on any protein, although they still have a specific point of attack.
Some enzymes work with only one substrate, others work with a group of molecules.
Succinic dehydrogenase oxidizes only succinic acid.
Proteases will act on any protein, although they still have a specific point of attack.
Слайд 13Enzyme-Catalyzed Reactions
Enzyme-catalyzed reactions are reversible.
Indicated by double arrows in reactions.
Tend to
go mostly in one direction.
Reactions tend to be catalyzed by different enzymes for each direction.
Catabolic (degradation) reaction catalyzed by enzyme A.
Anabolic (synthesis) reaction catalyzed by enzyme B.
Reactions tend to be catalyzed by different enzymes for each direction.
Catabolic (degradation) reaction catalyzed by enzyme A.
Anabolic (synthesis) reaction catalyzed by enzyme B.
Слайд 14Importance of ATP
Endergonic reactions require energy to proceed.
Coupling an energy-requiring reaction
with an energy-yielding reaction can drive endergonic reactions.
ATP is the most common intermediate in coupled reactions.
ATP is the most common intermediate in coupled reactions.
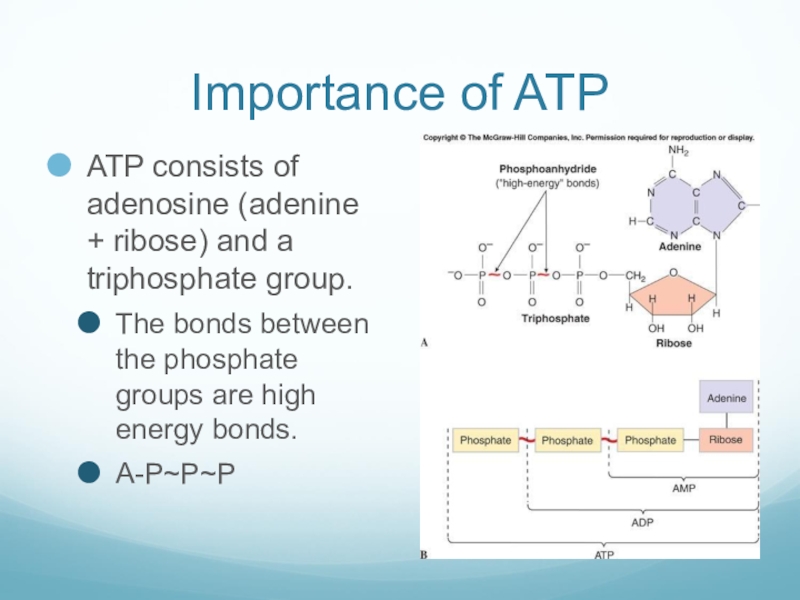
Слайд 15Importance of ATP
ATP consists of adenosine (adenine + ribose) and a
triphosphate group.
The bonds between the phosphate groups are high energy bonds.
A-P~P~P
The bonds between the phosphate groups are high energy bonds.
A-P~P~P
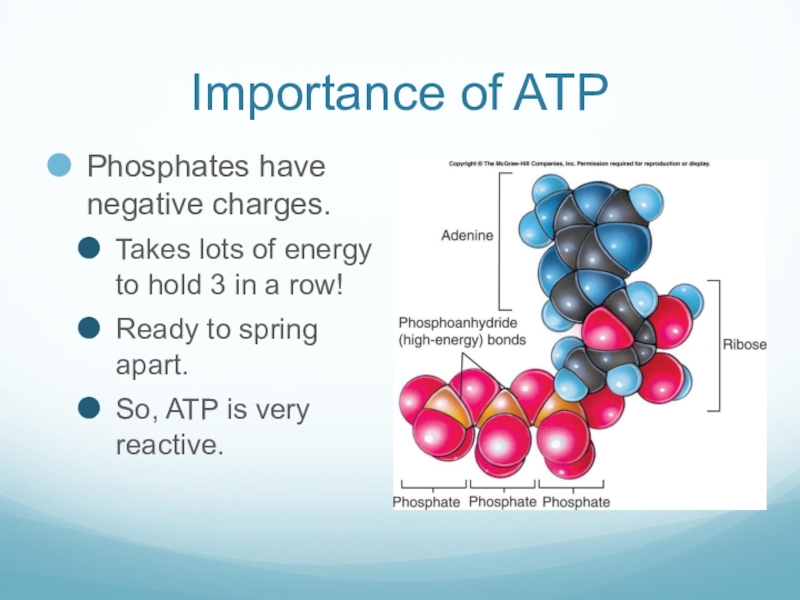
Слайд 16Importance of ATP
Phosphates have negative charges.
Takes lots of energy to hold
3 in a row!
Ready to spring apart.
So, ATP is very reactive.
Ready to spring apart.
So, ATP is very reactive.
Слайд 17Importance of ATP
A coupled reaction is a system of two reactions
linked by an energy shuttle – ATP.
Substrate B is a fuel – like glucose or lipid.
ATP is not a storehouse of energy – used as soon as it’s available.
Substrate B is a fuel – like glucose or lipid.
ATP is not a storehouse of energy – used as soon as it’s available.
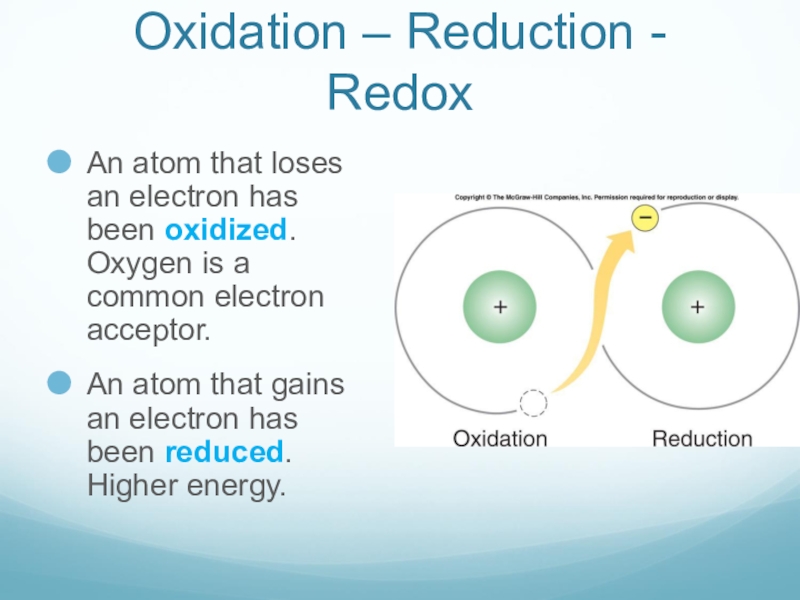
Слайд 18Oxidation – Reduction - Redox
An atom that loses an electron has
been oxidized. Oxygen is a common electron acceptor.
An atom that gains an electron has been reduced. Higher energy.
An atom that gains an electron has been reduced. Higher energy.
Слайд 19Redox Reactions
Redox reactions always occur in pairs.
One atom loses the electron,
the other gains the electron.
Energy is transferred from one atom to another via redox reactions.
Energy is transferred from one atom to another via redox reactions.
Слайд 20Cellular Respiration
Cellular respiration – the oxidation of food molecules to obtain
energy.
Electrons are stripped away.
Different from breathing (respiration).
Electrons are stripped away.
Different from breathing (respiration).
Слайд 21Cellular Respiration
Aerobic versus Anaerobic Metabolism
Heterotrophs
Aerobes: Use molecular oxygen as the final
electron acceptor
Anaerobes: Use other molecules as final electron acceptor
Energy yield much lower ATP yield
Anaerobes: Use other molecules as final electron acceptor
Energy yield much lower ATP yield
Слайд 22Cellular Respiration
When oxygen acts as the final electron acceptor (aerobes):
Almost 20
times more energy is released than if another acceptor is used (anaerobes).
Advantage of aerobic metabolism:
Smaller quantity of food required to maintain given rate of metabolism.
Advantage of aerobic metabolism:
Smaller quantity of food required to maintain given rate of metabolism.
Слайд 23Aerobic Respiration
In aerobic respiration, ATP forms as electrons are harvested, transferred
along the electron transport chain and eventually donated to O2 gas.
Oxygen is required!
Glucose is completely oxidized.
C6H12O6 + 6O2 6CO2 + 6H2O + energy (heat Glucose Oxygen Carbon Water or ATP)
Dioxide
Oxygen is required!
Glucose is completely oxidized.
C6H12O6 + 6O2 6CO2 + 6H2O + energy (heat Glucose Oxygen Carbon Water or ATP)
Dioxide
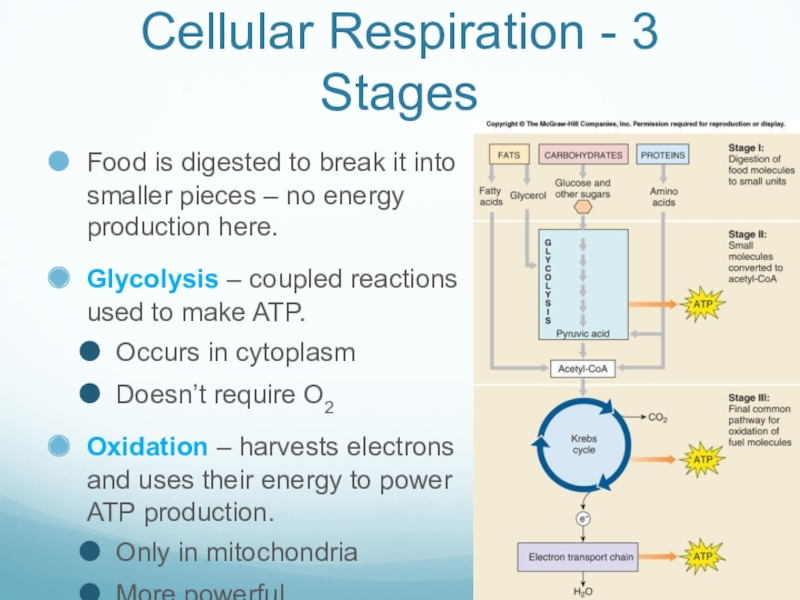
Слайд 24Cellular Respiration - 3 Stages
Food is digested to break it into
smaller pieces – no energy production here.
Glycolysis – coupled reactions used to make ATP.
Occurs in cytoplasm
Doesn’t require O2
Oxidation – harvests electrons and uses their energy to power ATP production.
Only in mitochondria
More powerful
Glycolysis – coupled reactions used to make ATP.
Occurs in cytoplasm
Doesn’t require O2
Oxidation – harvests electrons and uses their energy to power ATP production.
Only in mitochondria
More powerful
Слайд 25Anaerobic Respiration
Anaerobic respiration occurs in the absence of oxygen.
Different electron acceptors
are used instead of oxygen (sulfur, or nitrate).
Sugars are not completely oxidized, so it doesn’t generate as much ATP.
Sugars are not completely oxidized, so it doesn’t generate as much ATP.
Слайд 26Glycolysis
Glycolysis – the first stage in cellular respiration.
A series of enzyme
catalyzed reactions.
Glucose converted to pyruvic acid.
Small number of ATPs made (2 per glucose molecule), but it is possible in the absence of oxygen.
All living organisms use glycolysis.
Glucose converted to pyruvic acid.
Small number of ATPs made (2 per glucose molecule), but it is possible in the absence of oxygen.
All living organisms use glycolysis.
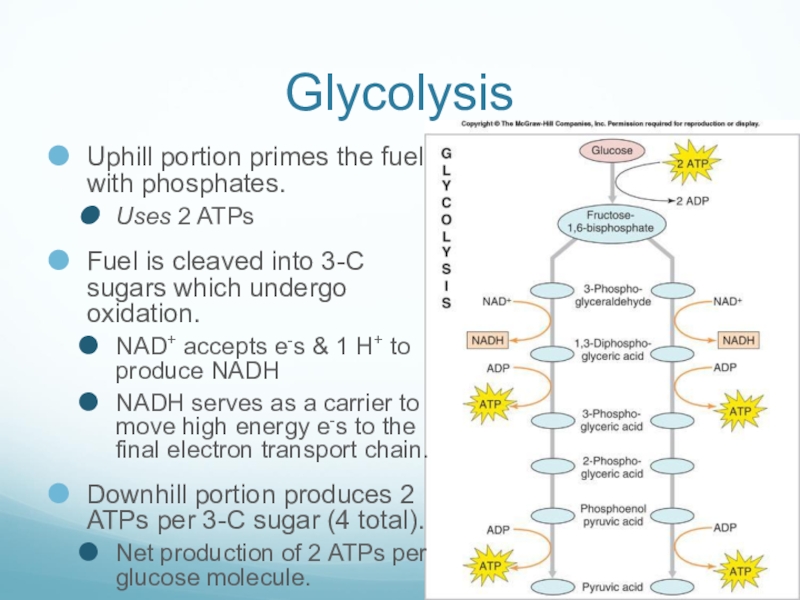
Слайд 27Glycolysis
Uphill portion primes the fuel with phosphates.
Uses 2 ATPs
Fuel is cleaved
into 3-C sugars which undergo oxidation.
NAD+ accepts e-s & 1 H+ to produce NADH
NADH serves as a carrier to move high energy e-s to the final electron transport chain.
Downhill portion produces 2 ATPs per 3-C sugar (4 total).
Net production of 2 ATPs per glucose molecule.
NAD+ accepts e-s & 1 H+ to produce NADH
NADH serves as a carrier to move high energy e-s to the final electron transport chain.
Downhill portion produces 2 ATPs per 3-C sugar (4 total).
Net production of 2 ATPs per glucose molecule.
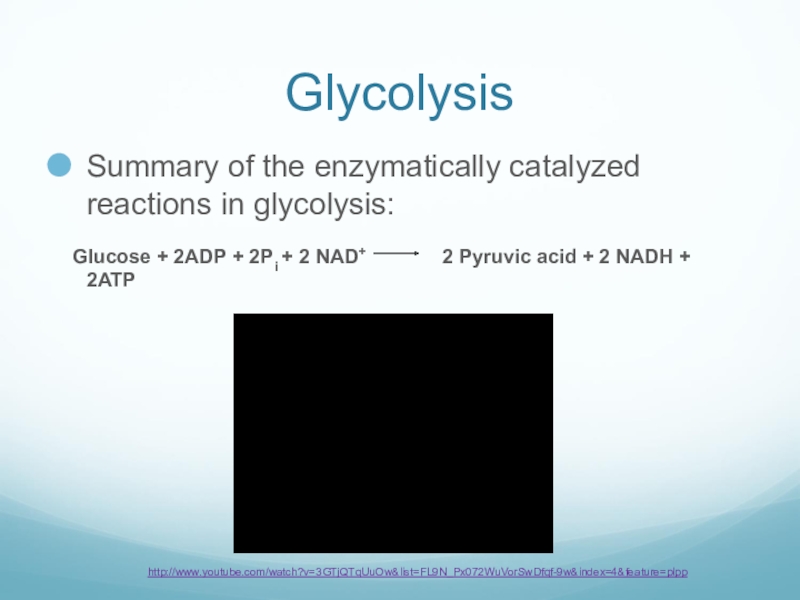
Слайд 28Glycolysis
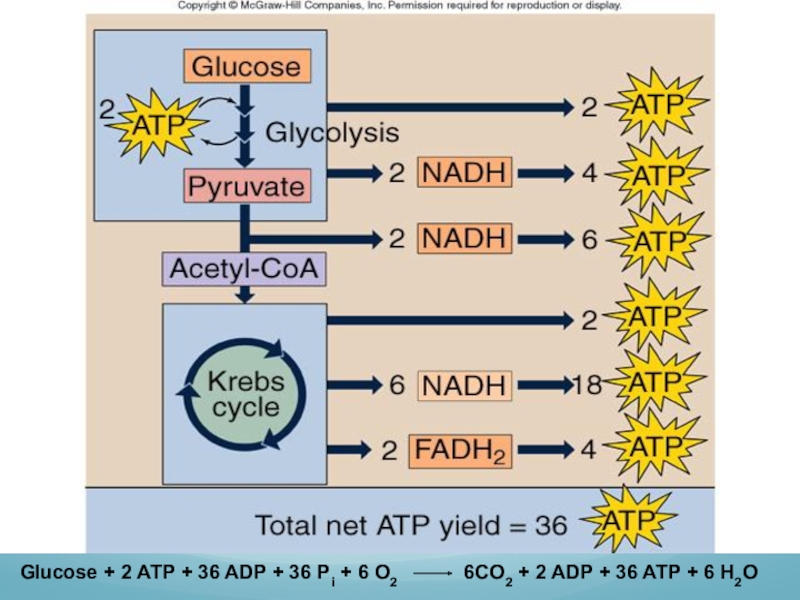
Summary of the enzymatically catalyzed reactions in glycolysis:
Glucose +
2ADP + 2Pi + 2 NAD+ 2 Pyruvic acid + 2 NADH + 2ATP
http://www.youtube.com/watch?v=3GTjQTqUuOw&list=FL9N_Px072WuVorSwDfqf-9w&index=4&feature=plpp
Слайд 29Harvesting Electrons form Chemical Bonds
When oxygen is available, a second oxidative
stage of cellular respiration takes place.
First step – oxidize the 3-carbon pyruvate in the mitochondria forming Acetyl-CoA.
Next, Acetyl-CoA is oxidized in the Krebs cycle.
First step – oxidize the 3-carbon pyruvate in the mitochondria forming Acetyl-CoA.
Next, Acetyl-CoA is oxidized in the Krebs cycle.
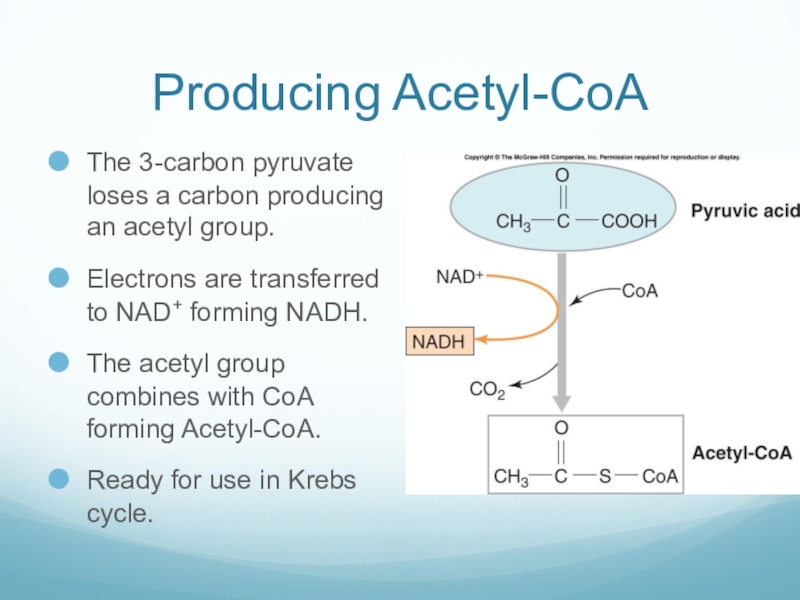
Слайд 30Producing Acetyl-CoA
The 3-carbon pyruvate loses a carbon producing an acetyl group.
Electrons
are transferred to NAD+ forming NADH.
The acetyl group combines with CoA forming Acetyl-CoA.
Ready for use in Krebs cycle.
The acetyl group combines with CoA forming Acetyl-CoA.
Ready for use in Krebs cycle.
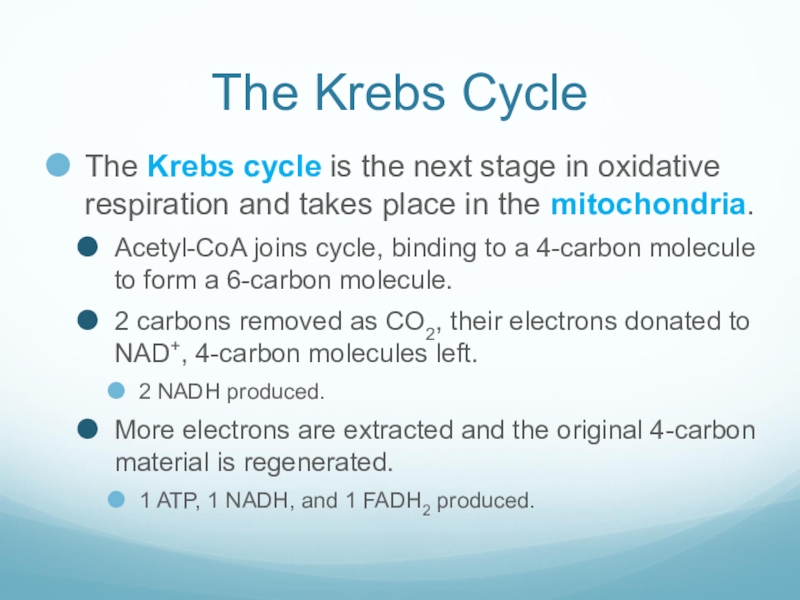
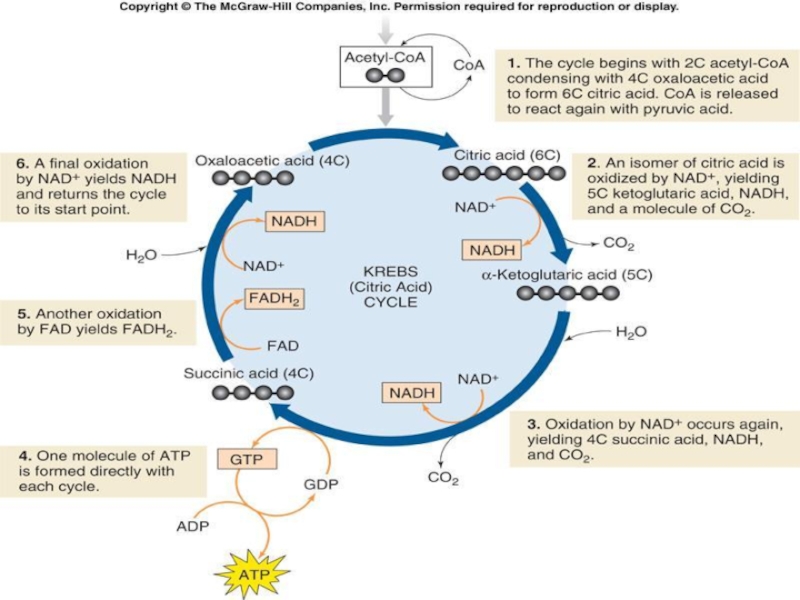
Слайд 31The Krebs Cycle
The Krebs cycle is the next stage in oxidative
respiration and takes place in the mitochondria.
Acetyl-CoA joins cycle, binding to a 4-carbon molecule to form a 6-carbon molecule.
2 carbons removed as CO2, their electrons donated to NAD+, 4-carbon molecules left.
2 NADH produced.
More electrons are extracted and the original 4-carbon material is regenerated.
1 ATP, 1 NADH, and 1 FADH2 produced.
Acetyl-CoA joins cycle, binding to a 4-carbon molecule to form a 6-carbon molecule.
2 carbons removed as CO2, their electrons donated to NAD+, 4-carbon molecules left.
2 NADH produced.
More electrons are extracted and the original 4-carbon material is regenerated.
1 ATP, 1 NADH, and 1 FADH2 produced.
Слайд 33The Krebs Cycle
Each glucose provides 2 pyruvates, therefore 2 turns of
the Krebs cycle.
Glucose is completely consumed during cellular respiration.
Glucose is completely consumed during cellular respiration.
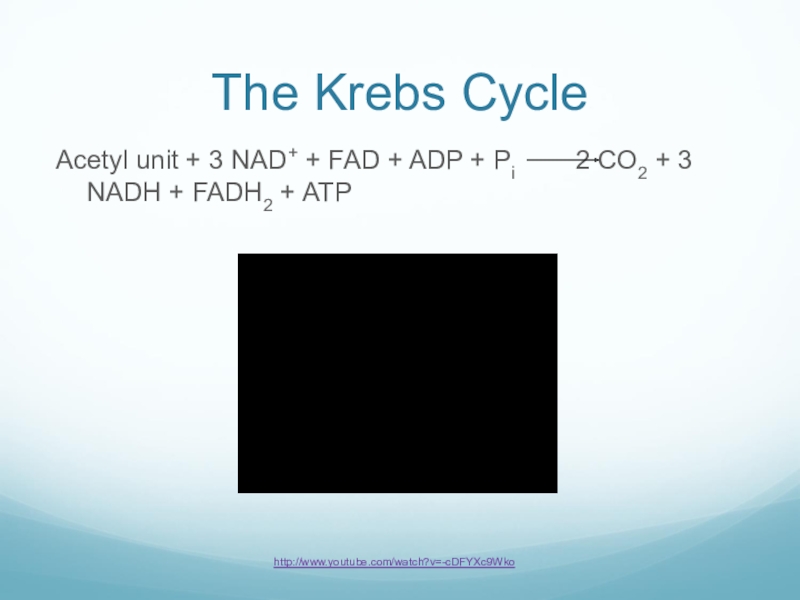
Слайд 34The Krebs Cycle
Acetyl unit + 3 NAD+ + FAD + ADP
+ Pi 2 CO2 + 3 NADH + FADH2 + ATP
http://www.youtube.com/watch?v=-cDFYXc9Wko
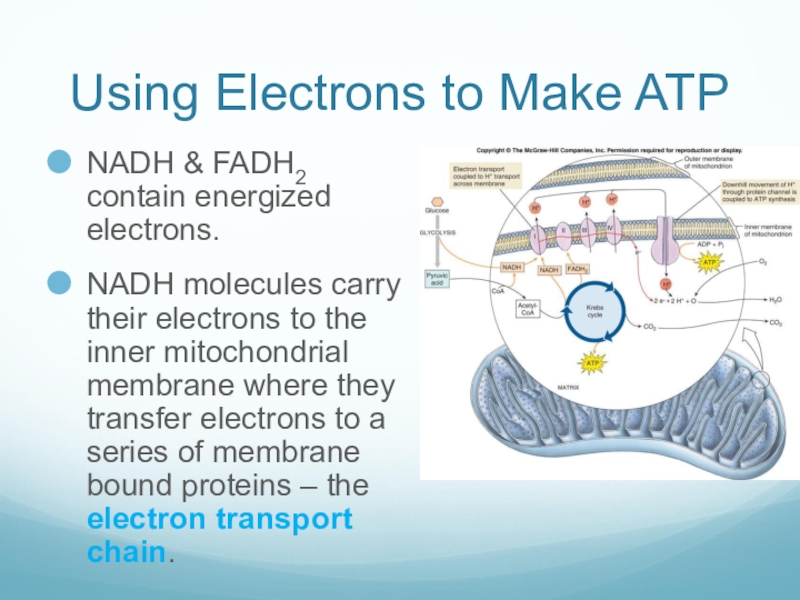
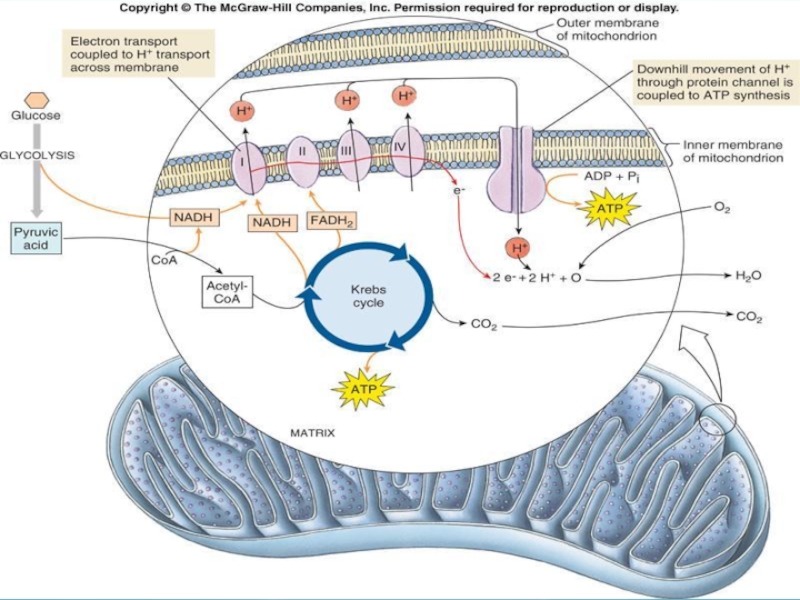
Слайд 35Using Electrons to Make ATP
NADH & FADH2 contain energized electrons.
NADH molecules
carry their electrons to the inner mitochondrial membrane where they transfer electrons to a series of membrane bound proteins – the electron transport chain.
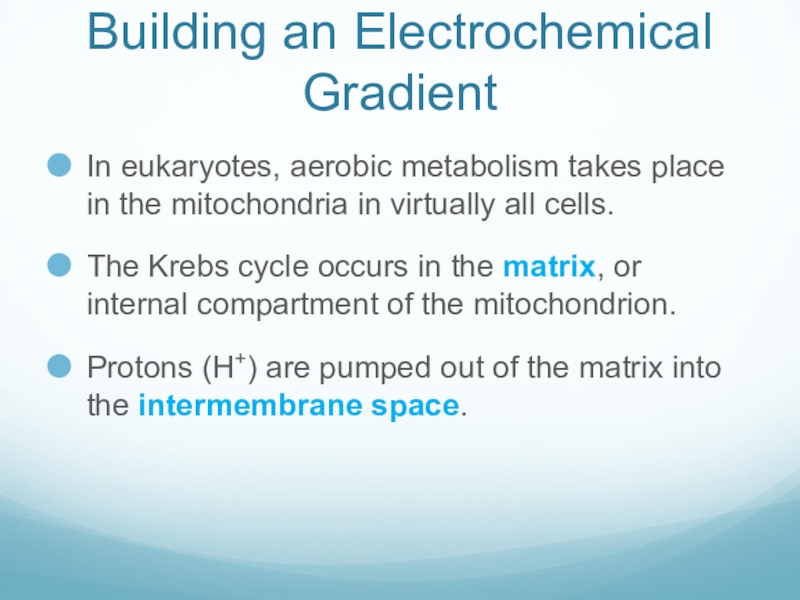
Слайд 36Building an Electrochemical Gradient
In eukaryotes, aerobic metabolism takes place in the
mitochondria in virtually all cells.
The Krebs cycle occurs in the matrix, or internal compartment of the mitochondrion.
Protons (H+) are pumped out of the matrix into the intermembrane space.
The Krebs cycle occurs in the matrix, or internal compartment of the mitochondrion.
Protons (H+) are pumped out of the matrix into the intermembrane space.
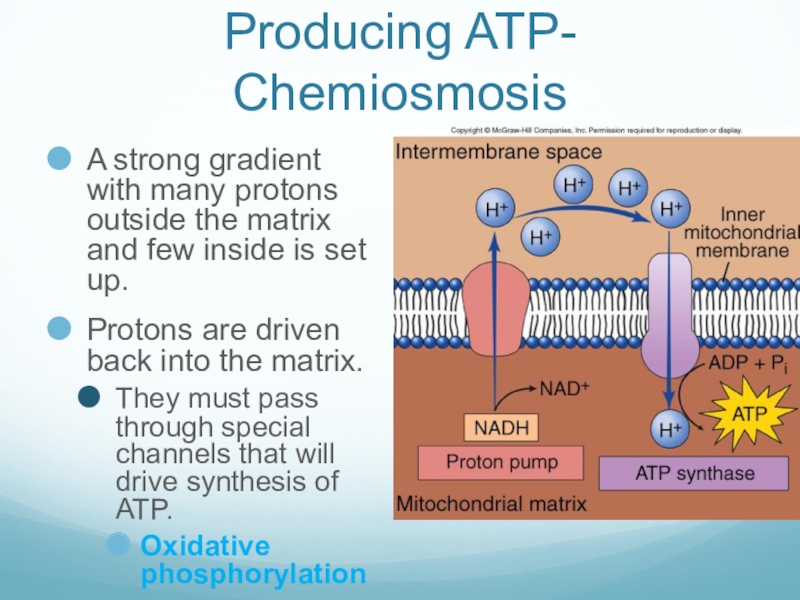
Слайд 37Producing ATP- Chemiosmosis
A strong gradient with many protons outside the matrix
and few inside is set up.
Protons are driven back into the matrix.
They must pass through special channels that will drive synthesis of ATP.
Oxidative phosphorylation
Protons are driven back into the matrix.
They must pass through special channels that will drive synthesis of ATP.
Oxidative phosphorylation
Слайд 39Electron Transport Review
http://www.youtube.com/watch?v=kN5MtqAB_Yc&list=FL9N_Px072WuVorSwDfqf-9w&index=2&feature=plpp
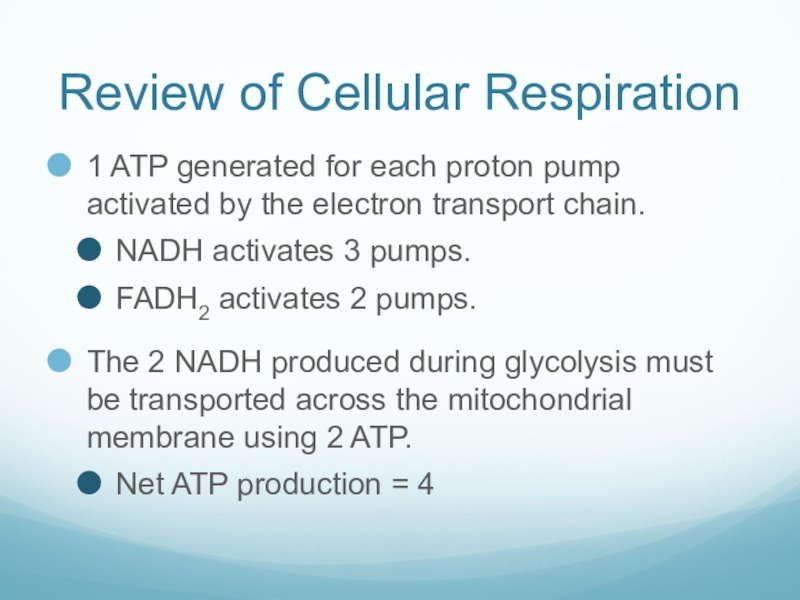
Слайд 40Review of Cellular Respiration
1 ATP generated for each proton pump activated
by the electron transport chain.
NADH activates 3 pumps.
FADH2 activates 2 pumps.
The 2 NADH produced during glycolysis must be transported across the mitochondrial membrane using 2 ATP.
Net ATP production = 4
NADH activates 3 pumps.
FADH2 activates 2 pumps.
The 2 NADH produced during glycolysis must be transported across the mitochondrial membrane using 2 ATP.
Net ATP production = 4
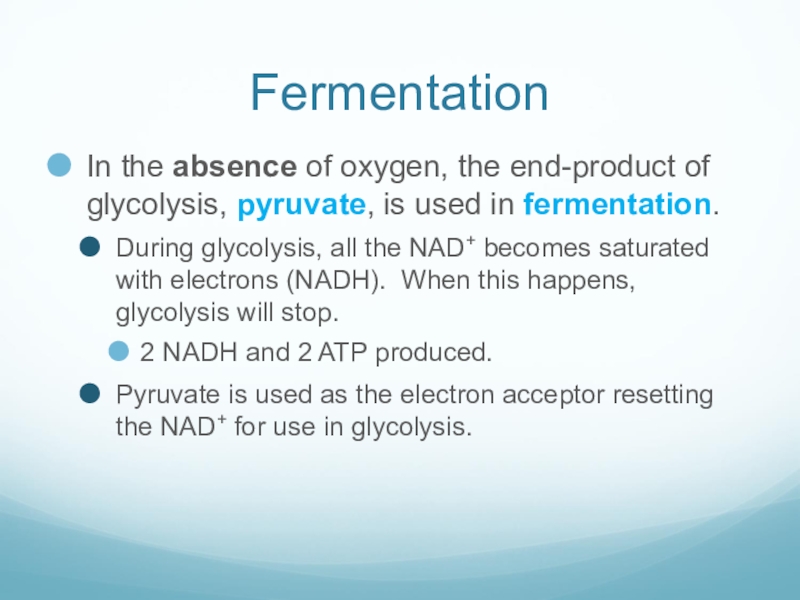
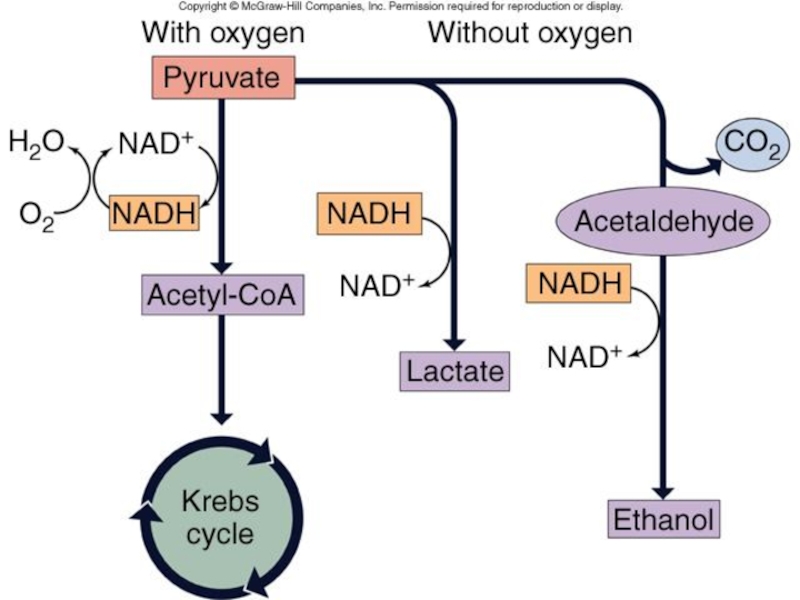
Слайд 42Fermentation
In the absence of oxygen, the end-product of glycolysis, pyruvate, is
used in fermentation.
During glycolysis, all the NAD+ becomes saturated with electrons (NADH). When this happens, glycolysis will stop.
2 NADH and 2 ATP produced.
Pyruvate is used as the electron acceptor resetting the NAD+ for use in glycolysis.
During glycolysis, all the NAD+ becomes saturated with electrons (NADH). When this happens, glycolysis will stop.
2 NADH and 2 ATP produced.
Pyruvate is used as the electron acceptor resetting the NAD+ for use in glycolysis.
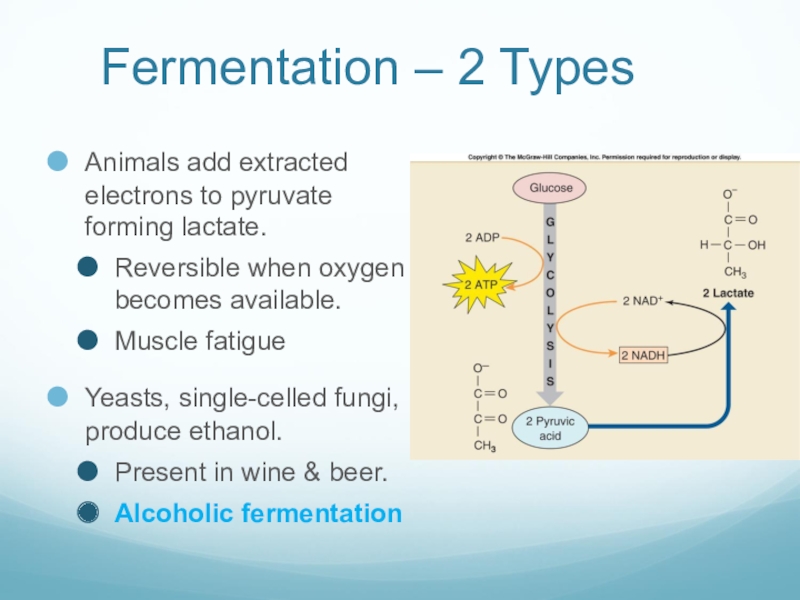
Слайд 43Fermentation – 2 Types
Animals add extracted electrons to pyruvate forming lactate.
Reversible
when oxygen becomes available.
Muscle fatigue
Yeasts, single-celled fungi, produce ethanol.
Present in wine & beer.
Alcoholic fermentation
Muscle fatigue
Yeasts, single-celled fungi, produce ethanol.
Present in wine & beer.
Alcoholic fermentation
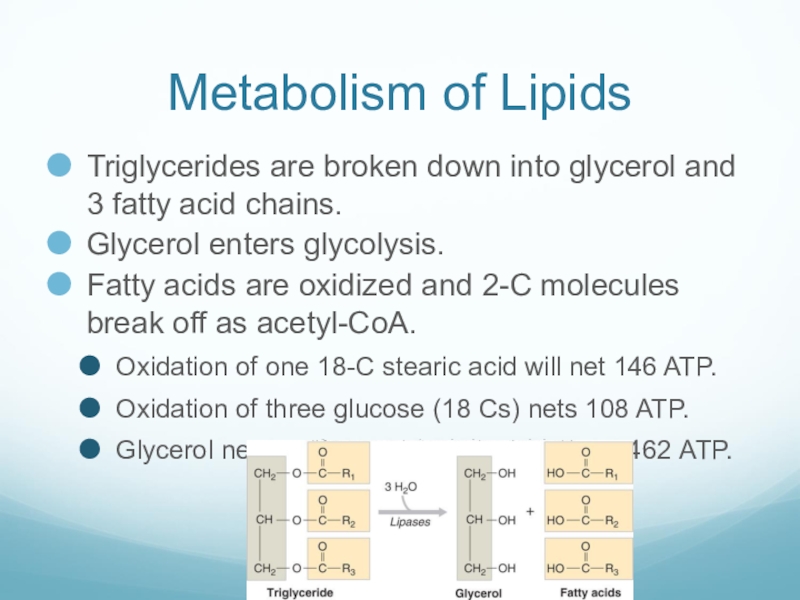
Слайд 45Metabolism of Lipids
Triglycerides are broken down into glycerol and 3 fatty
acid chains.
Glycerol enters glycolysis.
Fatty acids are oxidized and 2-C molecules break off as acetyl-CoA.
Oxidation of one 18-C stearic acid will net 146 ATP.
Oxidation of three glucose (18 Cs) nets 108 ATP.
Glycerol nets 22 ATP, so 1 triglyceride nets 462 ATP.
Glycerol enters glycolysis.
Fatty acids are oxidized and 2-C molecules break off as acetyl-CoA.
Oxidation of one 18-C stearic acid will net 146 ATP.
Oxidation of three glucose (18 Cs) nets 108 ATP.
Glycerol nets 22 ATP, so 1 triglyceride nets 462 ATP.
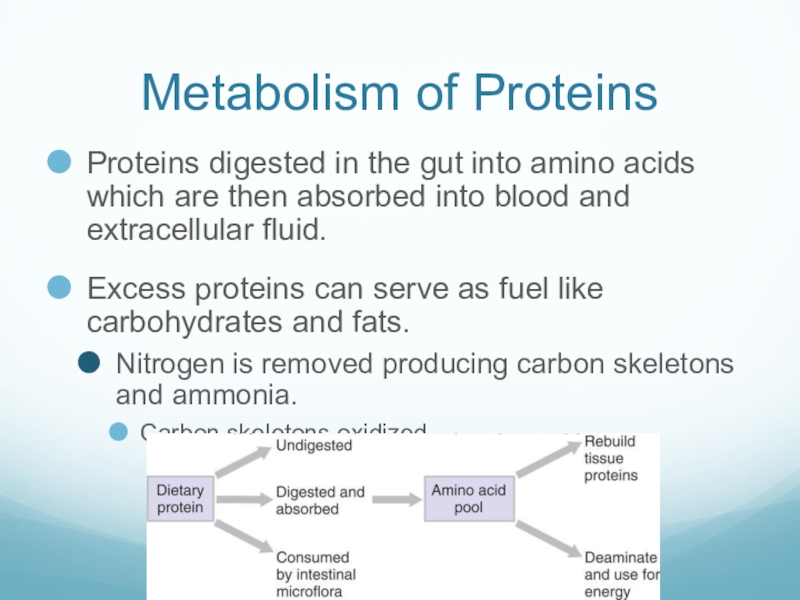
Слайд 46Metabolism of Proteins
Proteins digested in the gut into amino acids which
are then absorbed into blood and extracellular fluid.
Excess proteins can serve as fuel like carbohydrates and fats.
Nitrogen is removed producing carbon skeletons and ammonia.
Carbon skeletons oxidized.
Excess proteins can serve as fuel like carbohydrates and fats.
Nitrogen is removed producing carbon skeletons and ammonia.
Carbon skeletons oxidized.
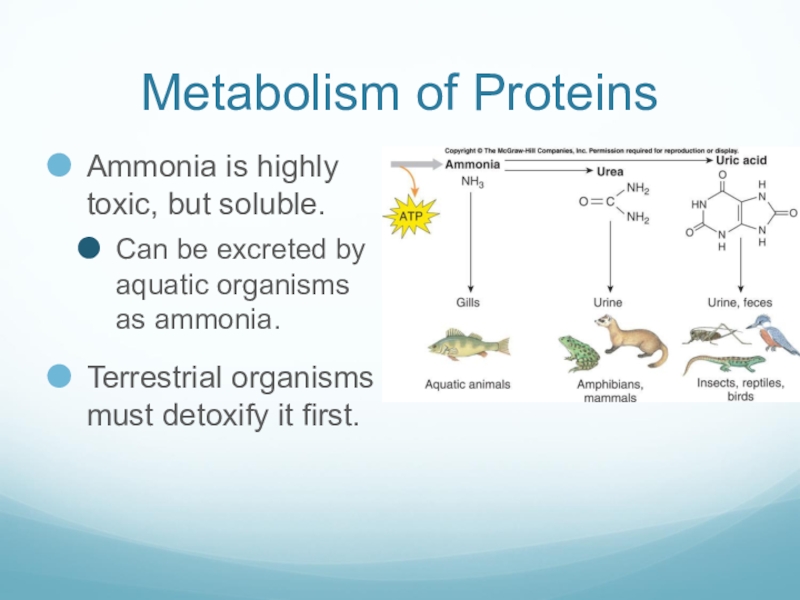
Слайд 47Metabolism of Proteins
Ammonia is highly toxic, but soluble.
Can be excreted by
aquatic organisms as ammonia.
Terrestrial organisms must detoxify it first.
Terrestrial organisms must detoxify it first.
Слайд 48Regulating Cellular Respiration
Rate of cellular respiration slows down when your cells
have enough ATP.
Enzymes that are important early in the process have an allosteric (regulating) site that will bind to ATP.
When lots of ATP is present, it will bind to this site, changing the shape of the enzyme, halting cellular respiration.
Enzymes that are important early in the process have an allosteric (regulating) site that will bind to ATP.
When lots of ATP is present, it will bind to this site, changing the shape of the enzyme, halting cellular respiration.
Слайд 49Regulating Cellular Respiration
Enzyme activity is controlled by presence or absence of
metabolites that cause conformational changes in enzymes.
Improves or decreases effectiveness as catalyst.
Improves or decreases effectiveness as catalyst.